Inhibition of Paracoccidioides lutzii Pb01 isocitrate lyase by the natural compound argentilactone and its semi-synthetic derivatives
- PMID: 24752170
- PMCID: PMC3994062
- DOI: 10.1371/journal.pone.0094832
Inhibition of Paracoccidioides lutzii Pb01 isocitrate lyase by the natural compound argentilactone and its semi-synthetic derivatives
Abstract
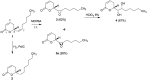
The dimorphic fungus Paracoccidioides spp. is responsible for paracoccidioidomycosis, the most prevalent systemic mycosis in Latin America, causing serious public health problems. Adequate treatment of mycotic infections is difficult, since fungi are eukaryotic organisms with a structure and metabolism similar to those of eukaryotic hosts. In this way, specific fungus targets have become important to search of new antifungal compound. The role of the glyoxylate cycle and its enzymes in microbial virulence has been reported in many fungal pathogens, including Paracoccidioides spp. Here, we show the action of argentilactone and its semi-synthetic derivative reduced argentilactone on recombinant and native isocitrate lyase from Paracoccidioides lutzii Pb01 (PbICL) in the presence of different carbon sources, acetate and glucose. Additionally, argentilactone and its semi-synthetic derivative reduced argentilactone exhibited relevant inhibitory activity against P. lutzii Pb01 yeast cells and dose-dependently influenced the transition from the mycelium to yeast phase. The other oxygenated derivatives tested, epoxy argentilactone and diol argentilactone-, did not show inhibitory action on the fungus. The results were supported by in silico experiments.
Conflict of interest statement
Figures




References
-
- Nunura RJ, Salazar MD, Vásquez LT, Endo GS, Rodríguez FA, et al. (2010) Paracoccidioidomicosis and multidrug-resistant tuberculosis (TBC-MDR) in patient coinfected with HIV and hepatitis C. Rev. Chilena Infectol. 27: 551–555 doi:/S0716-10182010000700011 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

