Lack of antimicrobial bactericidal activity in Mycobacterium abscessus
- PMID: 24752273
- PMCID: PMC4068550
- DOI: 10.1128/AAC.02448-14
Lack of antimicrobial bactericidal activity in Mycobacterium abscessus
Abstract
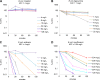
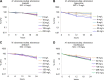
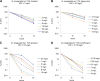
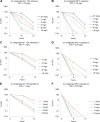
Antibiotic therapy of infections caused by the emerging pathogen Mycobacterium abscessus is challenging due to the organism's natural resistance toward most clinically available antimicrobials. We investigated the bactericidal activity of antibiotics commonly administered in M. abscessus infections in order to better understand the poor therapeutic outcome. Time-kill curves were generated for clinical M. abscessus isolates, Mycobacterium smegmatis, and Escherichia coli by using antibiotics commonly categorized as bactericidal (amikacin and moxifloxacin) or bacteriostatic (tigecycline and linezolid). In addition, the impact of aminoglycoside-modifying enzymes on the mode of action of substrate and nonsubstrate aminoglycosides was studied by using M. smegmatis as a model organism. While amikacin and moxifloxacin were bactericidal against E. coli, none of the tested compounds showed bactericidal activity against M. abscessus. Further mechanistic investigations of the mode of action of aminoglycosides in M. smegmatis revealed that the bactericidal activity of tobramycin and gentamicin was restored by disruption of the chromosomal aac(2') gene in the mycobacterial genome. The lack of bactericidal antibiotics in currently recommended treatment regimens provides a reasonable explanation for the poor therapeutic outcome in M. abscessus infection. Our findings suggest that chromosomally encoded drug-modifying enzymes play an important role in the lack of aminoglycoside bactericidal activity against rapidly growing mycobacteria.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures

References
-
- Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ, Jr, Winthrop K. 2007. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 175:367–416. 10.1164/rccm.200604-571ST - DOI - PubMed
-
- Bryant JM, Grogono DM, Greaves D, Foweraker J, Roddick I, Inns T, Reacher M, Haworth CS, Curran MD, Harris SR, Peacock SJ, Parkhill J, Floto RA. 2013. Whole-genome sequencing to identify transmission of Mycobacterium abscessus between patients with cystic fibrosis: a retrospective cohort study. Lancet 381:1551–1560. 10.1016/S0140-6736(13)60632-7 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

