Leucine-rich repeat containing 8A (LRRC8A) is essential for T lymphocyte development and function
- PMID: 24752297
- PMCID: PMC4010910
- DOI: 10.1084/jem.20131379
Leucine-rich repeat containing 8A (LRRC8A) is essential for T lymphocyte development and function
Abstract
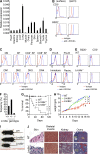
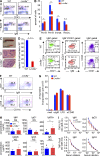
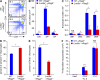
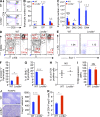
Lrrc8a is a ubiquitously expressed gene that encodes a leucine-rich repeat (LRR)-containing protein detected at higher levels on the surface of thymocytes than on other immune cells. We generated Lrrc8a(-/-) mice to investigate the role of LRRC8A in lymphocyte development and function. Lrrc8a(-/-) mice had increased prenatal and postnatal mortality, growth retardation, and multiple tissue abnormalities. Lrrc8a(-/-) mice displayed a modest block in B cell development but intact intrinsic B cell function. In contrast, both Lrrc8a(-/-) mice and Lrrc8a(-/-)→Rag2(-/-) bone marrow chimeras exhibited a severe cell-intrinsic block in early thymic development, with decreased proliferation and increased apoptosis of thymocytes, and impaired peripheral T cell function. Thymic epithelial cells expressed an LRRC8A ligand that was critical for double-negative to double-positive thymocyte differentiation and survival in vitro. LRRC8A constitutively associated with the GRB2-GAB2 complex and lymphocyte-specific protein tyrosine kinase (LCK) in thymocytes. LRRC8A ligation activated AKT via the LCK-ZAP-70-GAB2-PI3K pathway, and AKT phosphorylation was markedly reduced in the thymus of Lrrc8a(-/-) mice. These findings reveal an essential role for LRRC8A in T cell development, survival, and function.
Figures





Comment in
-
New role identified for LRR-containing proteins in T cell development.J Exp Med. 2014 May 5;211(5):746-7. doi: 10.1084/jem.2115insight4. J Exp Med. 2014. PMID: 24799532 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

