Measuring membrane protein bond orientations in nanodiscs via residual dipolar couplings
- PMID: 24752984
- PMCID: PMC4088969
- DOI: 10.1002/pro.2482
Measuring membrane protein bond orientations in nanodiscs via residual dipolar couplings
Abstract
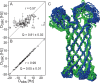
Membrane proteins are involved in numerous vital biological processes. To understand membrane protein functionality, accurate structural information is required. Usually, structure determination and dynamics of membrane proteins are studied in micelles using either solution state NMR or X-ray crystallography. Even though invaluable information has been obtained by this approach, micelles are known to be far from ideal mimics of biological membranes often causing the loss or decrease of membrane protein activity. Recently, nanodiscs, which are composed of a lipid bilayer surrounded by apolipoproteins, have been introduced as a more physiological alternative than micelles for NMR investigations on membrane proteins. Here, we show that membrane protein bond orientations in nanodiscs can be obtained by measuring residual dipolar couplings (RDCs) with the outer membrane protein OmpX embedded in nanodiscs using Pf1 phage as an alignment medium. The presented collection of membrane protein RDCs in nanodiscs represents an important step toward more comprehensive structural and dynamical NMR-based investigations of membrane proteins in a natural bilayer environment.
Keywords: COCAINE; NMR; Pf1 phage; TROSY; alignment medium; membrane protein; nanodisc; residual dipolar coupling.
© 2014 The Protein Society.
Figures

References
-
- Sanders CR, Landis GC. Reconstitution of membrane proteins into lipid-rich bilayered mixed micelles for NMR studies. Biochemistry. 1995;34:4030–4040. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

