Rabies virus envelope glycoprotein targets lentiviral vectors to the axonal retrograde pathway in motor neurons
- PMID: 24753246
- PMCID: PMC4047386
- DOI: 10.1074/jbc.M114.549980
Rabies virus envelope glycoprotein targets lentiviral vectors to the axonal retrograde pathway in motor neurons
Abstract
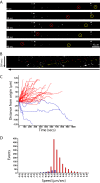
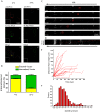
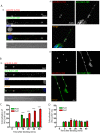
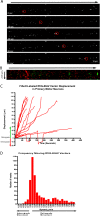
Rabies pseudotyped lentiviral vectors have great potential in gene therapy, not least because of their ability to transduce neurons following their distal axonal application. However, very little is known about the molecular processes that underlie their retrograde transport and cell transduction. Using multiple labeling techniques and confocal microscopy, we demonstrated that pseudotyping with rabies virus envelope glycoprotein (RV-G) enabled the axonal retrograde transport of two distinct subtypes of lentiviral vector in motor neuron cultures. Analysis of this process revealed that these vectors trafficked through Rab5-positive endosomes and accumulated within a non-acidic Rab7 compartment. RV-G pseudotyped vectors were co-transported with both the tetanus neurotoxin-binding fragment and the membrane proteins thought to mediate rabies virus endocytosis (neural cell adhesion molecule, nicotinic acetylcholine receptor, and p75 neurotrophin receptor), thus demonstrating that pseudotyping with RV-G targets lentiviral vectors for transport along the same pathway exploited by several toxins and viruses. Using motor neurons cultured in compartmentalized chambers, we demonstrated that axonal retrograde transport of these vectors was rapid and efficient; however, it was not able to transduce the targeted neurons efficiently, suggesting that impairment in processes occurring after arrival of the viral vector in the soma is responsible for the low transduction efficiency seen in vivo, which suggests a novel area for improvement of gene therapy vectors.
Keywords: Axon; Endosome; Glycoprotein; Lentivirus; Rab Proteins; Trafficking.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



References
-
- Wong L.-F., Goodhead L., Prat C., Mitrophanous K. A., Kingsman S. M., Mazarakis N. D. (2006) Lentivirus-mediated gene transfer to the central nervous system: therapeutic and research applications. Hum. Gene Ther. 17, 1–9 - PubMed
-
- Sakuma T., Barry M. A., Ikeda Y. (2012) Lentiviral vectors: basic to translational. Biochem. J. 443, 603–618 - PubMed
-
- Mazarakis N. D., Azzouz M., Rohll J. B., Ellard F. M., Wilkes F. J., Olsen A. L., Carter E. E., Barber R. D., Baban D. F., Kingsman S. M., Kingsman A. J., O'Malley K., Mitrophanous K. A. (2001) Rabies virus glycoprotein pseudotyping of lentiviral vectors enables retrograde axonal transport and access to the nervous system after peripheral delivery. Hum. Mol. Genet. 10, 2109–2121 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

