Visuospatial selective attention in chickens
- PMID: 24753566
- PMCID: PMC4024881
- DOI: 10.1073/pnas.1316824111
Visuospatial selective attention in chickens
Abstract
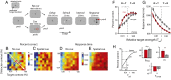
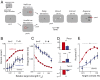
Voluntary control of attention promotes intelligent, adaptive behaviors by enabling the selective processing of information that is most relevant for making decisions. Despite extensive research on attention in primates, the capacity for selective attention in nonprimate species has never been quantified. Here we demonstrate selective attention in chickens by applying protocols that have been used to characterize visual spatial attention in primates. Chickens were trained to localize and report the vertical position of a target in the presence of task-relevant distracters. A spatial cue, the location of which varied across individual trials, indicated the horizontal, but not vertical, position of the upcoming target. Spatial cueing improved localization performance: accuracy (d') increased and reaction times decreased in a space-specific manner. Distracters severely impaired perceptual performance, and this impairment was greatly reduced by spatial cueing. Signal detection analysis with an "indecision" model demonstrated that spatial cueing significantly increased choice certainty in localizing targets. By contrast, error-aversion certainty (certainty of not making an error) remained essentially constant across cueing protocols, target contrasts, and individuals. The results show that chickens shift spatial attention rapidly and dynamically, following principles of stimulus selection that closely parallel those documented in primates. The findings suggest that the mechanisms that control attention have been conserved through evolution, and establish chickens--a highly visual species that is easily trained and amenable to cutting-edge experimental technologies--as an attractive model for linking behavior to neural mechanisms of selective attention.
Keywords: avian vision; competitive selection; executive functions; top-down control; voluntary attention.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

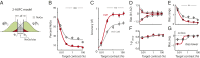

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

