Myosin-10 produces its power-stroke in two phases and moves processively along a single actin filament under low load
- PMID: 24753602
- PMCID: PMC4020102
- DOI: 10.1073/pnas.1320122111
Myosin-10 produces its power-stroke in two phases and moves processively along a single actin filament under low load
Abstract
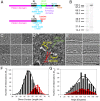
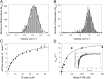
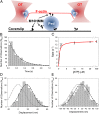
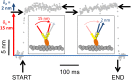
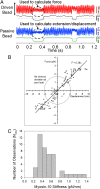
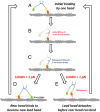
Myosin-10 is an actin-based molecular motor that participates in essential intracellular processes such as filopodia formation/extension, phagocytosis, cell migration, and mitotic spindle maintenance. To study this motor protein's mechano-chemical properties, we used a recombinant, truncated form of myosin-10 consisting of the first 936 amino acids, followed by a GCN4 leucine zipper motif, to force dimerization. Negative-stain electron microscopy reveals that the majority of molecules are dimeric with a head-to-head contour distance of ∼50 nm. In vitro motility assays show that myosin-10 moves actin filaments smoothly with a velocity of ∼310 nm/s. Steady-state and transient kinetic analysis of the ATPase cycle shows that the ADP release rate (∼13 s(-1)) is similar to the maximum ATPase activity (∼12-14 s(-1)) and therefore contributes to rate limitation of the enzymatic cycle. Single molecule optical tweezers experiments show that under intermediate load (∼0.5 pN), myosin-10 interacts intermittently with actin and produces a power stroke of ∼17 nm, composed of an initial 15-nm and subsequent 2-nm movement. At low optical trap loads, we observed staircase-like processive movements of myosin-10 interacting with the actin filament, consisting of up to six ∼35-nm steps per binding interaction. We discuss the implications of this load-dependent processivity of myosin-10 as a filopodial transport motor.
Keywords: actomyosin; myosin X; myosin-5a; optical trapping; stable single alpha-helix.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Richards TA, Cavalier-Smith T. Myosin domain evolution and the primary divergence of eukaryotes. Nature. 2005;436(7054):1113–1118. - PubMed
-
- Berg JS, Derfler BH, Pennisi CM, Corey DP, Cheney RE. Myosin-X, a novel myosin with pleckstrin homology domains, associates with regions of dynamic actin. J Cell Sci. 2000;113(Pt 19):3439–3451. - PubMed
-
- Berg JS, Cheney RE. Myosin-X is an unconventional myosin that undergoes intrafilopodial motility. Nat Cell Biol. 2002;4(3):246–250. - PubMed
-
- Zhu XJ, et al. Myosin X regulates netrin receptors and functions in axonal path-finding. Nat Cell Biol. 2007;9(2):184–192. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

