The uterine myocyte as a target for prevention of preterm birth
- PMID: 24753931
- PMCID: PMC3987349
The uterine myocyte as a target for prevention of preterm birth
Abstract
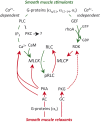
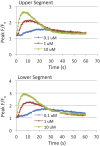
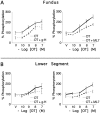
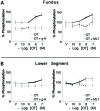
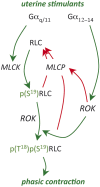
Preterm birth (PTB) remains the most common cause of neonatal morbidity and mortality as well as long-term disability. Current strategies to prevent or arrest spontaneous preterm labor (SPTL) have limited success. For almost three decades, there have been no novel pharmacological agents used clinically to address this important obstetrical complication. In this review, we focus on the uterine myocyte as a target for prevention of spontaneous PTB. After presenting an overview of intracellular signaling pathways that are important in regulation of smooth muscle contractility, we discuss previous and current pharmacological approaches to manage SPTL. We also present recent evidence from our own laboratories suggesting a potentially novel and uterine-specific approach to maintain or impose uterine relaxation. Finally, we briefly discuss extrinsic systems that might affect uterine activity and reinforce the concept that SPTL represents a syndrome that is the end result of a variety of pathophysiologic etiologies leading to PTB. We conclude by emphasizing the need for much more research to provide sufficient understanding of the mechanisms of SPTL and to make inroads towards reducing the incidence and adverse consequences of this common and serious syndrome.
Keywords: Prematurity; myosin regulatory light chain; preterm labour; rhoA-associated kinase; tocolysis; tocolysis contractility.
Figures

References
-
- Aguilar HN, Mitchell BF. Physiological Pathways and Molecular Mechanisms Regulating Uterine Contractility. Hum Reprod Update. 2010;16:725–744. - PubMed
-
- Aguilar HN, Tracey CN, Zielnik B, Mitchell BF. Rho-kinase mediates diphosphorylation of myosin regulatory light chain in cultured uterine but not vascular smooth muscle cells. http://onlinelibrary.wiley.com/doi/ 10.1111/j.1582-4934.2012.01625.x/abs... J Cell Mol Med. 2012 - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
