Persistence of pulmonary tertiary lymphoid tissues and anti-nuclear antibodies following cessation of cigarette smoke exposure
- PMID: 24754996
- PMCID: PMC4021094
- DOI: 10.1186/1465-9921-15-49
Persistence of pulmonary tertiary lymphoid tissues and anti-nuclear antibodies following cessation of cigarette smoke exposure
Abstract
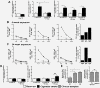
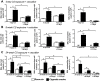
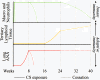
Formation of pulmonary tertiary immune structures is a characteristic feature of advanced COPD. In the current study, we investigated the mechanisms of tertiary lymphoid tissue (TLT) formation in the lungs of cigarette smoke-exposed mice. We found that cigarette smoke exposure led to TLT formation that persisted following smoking cessation. TLTs consisted predominantly of IgM positive B cells, while plasma cells in close proximity to TLTs expressed IgM, IgG, and IgA. The presence of TLT formation was associated with anti-nuclear autoantibody (ANA) production that also persisted following smoking cessation. ANAs were observed in the lungs, but not the circulation of cigarette smoke-exposed mice. Similarly, we observed ANA in the sputum of COPD patients where levels correlated with disease severity and were refractory to steroid treatment. Both ANA production and TLT formation were dependent on interleukin-1 receptor 1 (IL-1R1) expression. Contrary to TLT and ANA, lung neutrophilia resolved following smoking cessation. These data suggest a differential regulation of innate and B cell-related immune inflammatory processes associated with cigarette smoke exposure. Moreover, our study further emphasizes the importance of interleukin-1 (IL-1) signaling pathways in cigarette smoke-related pulmonary pathogenesis.
Figures



References
-
- U.S. Department of Health and Human Services. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2010.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

