Relationship between time in therapeutic range and comparative treatment effect of rivaroxaban and warfarin: results from the ROCKET AF trial
- PMID: 24755148
- PMCID: PMC4187517
- DOI: 10.1161/JAHA.113.000521
Relationship between time in therapeutic range and comparative treatment effect of rivaroxaban and warfarin: results from the ROCKET AF trial
Abstract
Background: Time in therapeutic range (TTR) is a standard quality measure of the use of warfarin. We assessed the relative effects of rivaroxaban versus warfarin at the level of trial center TTR (cTTR) since such analysis preserves randomized comparisons.
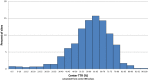
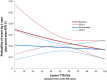
Methods and results: TTR was calculated using the Rosendaal method, without exclusion of international normalized ratio (INR) values performed during warfarin initiation. Measurements during warfarin interruptions >7 days were excluded. INRs were performed via standardized finger-stick point-of-care devices at least every 4 weeks. The primary efficacy endpoint (stroke or non-central nervous system embolism) was examined by quartiles of cTTR and by cTTR as a continuous function. Centers with the highest cTTRs by quartile had lower-risk patients as reflected by lower CHADS2 scores (P<0.0001) and a lower prevalence of prior stroke or transient ischemic attack (P<0.0001). Sites with higher cTTR were predominantly from North America and Western Europe. The treatment effect of rivaroxaban versus warfarin on the primary endpoint was consistent across a wide range of cTTRs (P value for interaction=0.71). The hazard of major and non-major clinically relevant bleeding increased with cTTR (P for interaction=0.001), however, the estimated reduction by rivaroxaban compared with warfarin in the hazard of intracranial hemorrhage was preserved across a wide range of threshold cTTR values.
Conclusions: The treatment effect of rivaroxaban compared with warfarin for the prevention of stroke and systemic embolism is consistent regardless of cTTR.
Keywords: rivaroxaban; time in therapeutic range; warfarin.
Figures


Similar articles
-
Impact of global geographic region on time in therapeutic range on warfarin anticoagulant therapy: data from the ROCKET AF clinical trial.J Am Heart Assoc. 2013 Feb 19;2(1):e000067. doi: 10.1161/JAHA.112.000067. J Am Heart Assoc. 2013. PMID: 23525418 Free PMC article. Clinical Trial.
-
Alternative calculations of individual patient time in therapeutic range while taking warfarin: results from the ROCKET AF trial.J Am Heart Assoc. 2015 Mar 3;4(3):e001349. doi: 10.1161/JAHA.114.001349. J Am Heart Assoc. 2015. PMID: 25736441 Free PMC article. Clinical Trial.
-
Outcomes of discontinuing rivaroxaban compared with warfarin in patients with nonvalvular atrial fibrillation: analysis from the ROCKET AF trial (Rivaroxaban Once-Daily, Oral, Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation).J Am Coll Cardiol. 2013 Feb 12;61(6):651-8. doi: 10.1016/j.jacc.2012.09.057. J Am Coll Cardiol. 2013. PMID: 23391196 Clinical Trial.
-
Rivaroxaban for stroke prevention in atrial fibrillation: a critical review of the ROCKET AF trial.Expert Rev Cardiovasc Ther. 2012 Aug;10(8):965-72. doi: 10.1586/erc.12.91. Expert Rev Cardiovasc Ther. 2012. PMID: 23030284 Review.
-
Rivaroxaban: a review of its use in the prevention of stroke and systemic embolism in patients with atrial fibrillation.Drugs. 2013 May;73(7):715-39. doi: 10.1007/s40265-013-0056-9. Drugs. 2013. PMID: 23677801 Review.
Cited by
-
How to choose appropriate direct oral anticoagulant for patient with nonvalvular atrial fibrillation.Ann Hematol. 2016 Feb;95(3):437-49. doi: 10.1007/s00277-015-2566-x. Epub 2015 Dec 11. Ann Hematol. 2016. PMID: 26658769 Free PMC article. Review.
-
Clinical and Biochemical Differences in Patients Having Non-Variceal Upper Gastrointestinal Bleeding on NSAIDs, Oral Anticoagulants, and Antiplatelet Therapy.J Clin Med. 2024 Sep 22;13(18):5622. doi: 10.3390/jcm13185622. J Clin Med. 2024. PMID: 39337109 Free PMC article.
-
Influence of renal insufficiency on anticoagulant effects and safety of warfarin in Chinese patients: analysis from a randomized controlled trial.Naunyn Schmiedebergs Arch Pharmacol. 2021 Jun;394(6):1275-1283. doi: 10.1007/s00210-020-02037-3. Epub 2021 Jan 6. Naunyn Schmiedebergs Arch Pharmacol. 2021. PMID: 33404689 Free PMC article. Clinical Trial.
-
Efficacy and Safety of Direct Oral Anticoagulants for Stroke Prevention in Older Patients With Atrial Fibrillation: A Network Meta-Analysis of Randomized Controlled Trials.J Am Heart Assoc. 2023 Dec 5;12(23):e030380. doi: 10.1161/JAHA.123.030380. Epub 2023 Nov 28. J Am Heart Assoc. 2023. PMID: 38014696 Free PMC article.
-
International normalized ratio control and subsequent clinical outcomes in patients with atrial fibrillation using warfarin.J Thromb Thrombolysis. 2019 Jul;48(1):27-34. doi: 10.1007/s11239-019-01858-1. J Thromb Thrombolysis. 2019. PMID: 30972712
References
-
- Jackson K, Gersh BJ, Stockbridge N, Fleming TR, Temple R, Califf RM, Connolly SJ, Wallentin L, Granger CB. Antithrombotic drug development for atrial fibrillation: proceedings, Washington, DC, July 25‐27, 2005. Am Heart J. 2008; 155:829-840 - PubMed
-
- Wan Y, Heneghan C, Perera R, Roberts N, Hollowell J, Glasziou P, Bankhead C, Xu Y. Anticoagulation control and prediction of adverse events in patients with atrial fibrillation: a systematic review. Circ Cardiovasc Qual Outcomes. 2008; 1:84-91 - PubMed
-
- Estes NA, III, Halperin JL, Calkins H, Ezekowitz MD, Gitman P, Go AS, McNamara RL, Messer JV, Ritchie JL, Romeo SJ, Waldo AL, Wyse DG. ACC/AHA/Physician Consortium 2008 clinical performance measures for adults with nonvalvular atrial fibrillation or atrial flutter. Circulation. 2008; 117:1101-1120 - PubMed
-
- Rosendaal FR, Cannegieter SC, van der Meer FJ, Briet E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993; 69:236-239 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

