AP39, a novel mitochondria-targeted hydrogen sulfide donor, stimulates cellular bioenergetics, exerts cytoprotective effects and protects against the loss of mitochondrial DNA integrity in oxidatively stressed endothelial cells in vitro
- PMID: 24755204
- PMCID: PMC4225488
- DOI: 10.1016/j.niox.2014.04.008
AP39, a novel mitochondria-targeted hydrogen sulfide donor, stimulates cellular bioenergetics, exerts cytoprotective effects and protects against the loss of mitochondrial DNA integrity in oxidatively stressed endothelial cells in vitro
Abstract
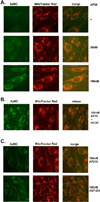
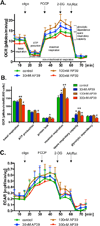
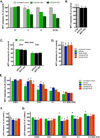
The purpose of the current study was to investigate the effect of the recently synthesized mitochondrially-targeted H2S donor, AP39 [(10-oxo-10-(4-(3-thioxo-3H-1,2-dithiol-5yl)phenoxy)decyl) triphenylphosphonium bromide], on bioenergetics, viability, and mitochondrial DNA integrity in bEnd.3 murine microvascular endothelial cells in vitro, under normal conditions, and during oxidative stress. Intracellular H2S was assessed by the fluorescent dye 7-azido-4-methylcoumarin. For the measurement of bioenergetic function, the XF24 Extracellular Flux Analyzer was used. Cell viability was estimated by the combination of the MTT and LDH methods. Oxidative protein modifications were measured by the Oxyblot method. Reactive oxygen species production was monitored by the MitoSOX method. Mitochondrial and nuclear DNA integrity were assayed by the Long Amplicon PCR method. Oxidative stress was induced by addition of glucose oxidase. Addition of AP39 (30-300 nM) to bEnd.3 cells increased intracellular H2S levels, with a preferential response in the mitochondrial regions. AP39 exerted a concentration-dependent effect on mitochondrial activity, which consisted of a stimulation of mitochondrial electron transport and cellular bioenergetic function at lower concentrations (30-100 nM) and an inhibitory effect at the higher concentration of 300 nM. Under oxidative stress conditions induced by glucose oxidase, an increase in oxidative protein modification and an enhancement in MitoSOX oxidation was noted, coupled with an inhibition of cellular bioenergetic function and a reduction in cell viability. AP39 pretreatment attenuated these responses. Glucose oxidase induced a preferential damage to the mitochondrial DNA; AP39 (100 nM) pretreatment protected against it. In conclusion, the current paper documents antioxidant and cytoprotective effects of AP39 under oxidative stress conditions, including a protection against oxidative mitochondrial DNA damage.
Keywords: Bioenergetics; Cytoprotection; DNA repair; Mitochondria; Oxidative stress.
Copyright © 2014 Elsevier Inc. All rights reserved.
Conflict of interest statement
MW, MEW and AP have filed for patent protection for AP39 WO2013045951 on 01/10/12.
Figures





References
-
- Szabo C. Hydrogen sulphide and its therapeutic potential. Nat. Rev. Drug Discov. 2007;6:917–935. - PubMed
-
- Whiteman M, Le Trionnaire S, Chopra M, Fox B, Whatmore J. Emerging role of hydrogen sulfide in health and disease: critical appraisal of biomarkers and pharmacological tools. Clin. Sci. (Lond) 2011;121:459–488. - PubMed
-
- Wang R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol. Rev. 2012;92:791–896. - PubMed
-
- Elrod JW, Calvert JW, Morrison J, Doeller JE, Kraus DW, Tao L, Jiao X, Scalia R, Kiss L, Szabo C, Kimura H, Chow CW, Lefer DJ. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc. Natl. Acad. Sci. USA. 2007;104:15560–15565. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

