Toll-like receptor 4 signaling contributes to Paclitaxel-induced peripheral neuropathy
- PMID: 24755282
- PMCID: PMC4083500
- DOI: 10.1016/j.jpain.2014.04.001
Toll-like receptor 4 signaling contributes to Paclitaxel-induced peripheral neuropathy
Abstract
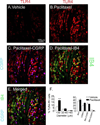
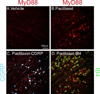
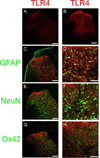
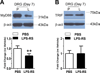
This paper tests the contribution of the toll-like receptors, TLR4 in particular, in the initiation and maintenance of paclitaxel-related chemotherapy-induced peripheral neuropathy. TLR4 and its immediate downstream signaling molecules-myeloid differentiation primary response gene 88 (MyD88) and toll/interleukin 1 receptor domain-containing adapter-inducing interferon-β (TRIF)-were found to be increased in the dorsal root ganglion (DRG) using Western blot by day 7 of paclitaxel treatment. The behavioral phenotype, the increase of both TLR4 and MyD88, was blocked by cotreatment with the TLR4 antagonist lipopolysaccharide-Rhodobacter sphaeroides during chemotherapy. A similar, but less robust, behavioral effect was observed using intrathecal treatment of MyD88 homodimerization inhibitory peptide. DRG levels of TLR4 and MyD88 reduced over the next 2 weeks, whereas these levels remained increased in spinal cord through day 21 following chemotherapy. Immunohistochemical analysis revealed TLR4 expression in both calcitonin gene-related peptide-positive and isolectin B4-positive small DRG neurons. MyD88 was only found in calcitonin gene-related peptide-positive neurons, and TRIF was found in both calcitonin gene-related peptide-positive and isolectin B4-positive small DRG neurons as well as in medium- and large-size DRG neurons. In the spinal cord, TLR4 was only found colocalized to astrocytes but not with either microglia or neurons. Intrathecal treatment with the TLR4 antagonist lipopolysaccharide-R. sphaeroides transiently reversed preestablished chemotherapy-induced peripheral neuropathy mechanical hypersensitivity. These results strongly implicate TLR4 signaling in the DRG and the spinal cord in the induction and maintenance of paclitaxel-related chemotherapy-induced peripheral neuropathy.
Perspective: The toll-like receptor TLR4 and MyD88 signaling pathway could be a new potential therapeutic target in paclitaxel-induced painful neuropathy.
Keywords: DRG; LPS-RS; MyD88; Neuropathy; TLR4; TRIF; spinal cord.
Copyright © 2014 American Pain Society. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Asehnoune K, Strassheim D, Mitra S, Kim JY, Abraham E. Involvement of reactive oxygen species in Toll-like receptor 4-dependent activation of NF-kappa B. J Immunol. 2004 Feb 15;172:2522–2529. - PubMed
-
- Balosso S, Liu J, Bianchi ME, Vezzani A. Disulfide-Containing High Mobility Group Box-1 Promotes N-Methyl-d-Aspartate Receptor Function and Excitotoxicity by Activating Toll-Like Receptor 4-Dependent Signaling in Hippocampal Neurons. Antioxid Redox Signal. 2014 Jan 3; - PubMed
-
- Basu S, Sodhi A. Increased release of interleukin-1 and tumour necrosis factor by interleukin-2-induced lymphokine-activated killer cells in the presence of cisplatin and FK-565. Immunol Cell Biol. 1992;70(Pt 1):15–24. - PubMed
-
- Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, von Zastrow M, Beattie MS, Malenka RC. Control of synaptic strength by glial TNFa. Science. 2002;295:2282–2285. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

