Increased susceptibility to vaginal simian/human immunodeficiency virus transmission in pig-tailed macaques coinfected with Chlamydia trachomatis and Trichomonas vaginalis
- PMID: 24755433
- PMCID: PMC4271071
- DOI: 10.1093/infdis/jiu240
Increased susceptibility to vaginal simian/human immunodeficiency virus transmission in pig-tailed macaques coinfected with Chlamydia trachomatis and Trichomonas vaginalis
Abstract
Background: Sexually transmitted infections (STIs) are associated with an increased risk of human immunodeficiency virus (HIV) infection, but their biological effect on HIV susceptibility is not fully understood.
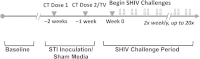
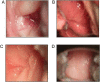
Methods: Female pig-tailed macaques inoculated with Chlamydia trachomatis and Trichomonas vaginalis (n = 9) or medium (controls; n = 7) were repeatedly challenged intravaginally with SHIVSF162p3. Virus levels were evaluated by real-time polymerase chain reaction, plasma and genital cytokine levels by Luminex assays, and STI clinical signs by colposcopy.
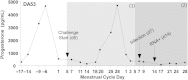
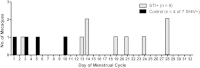
Results: Simian/HIV (SHIV) susceptibility was enhanced in STI-positive macaques (P = .04, by the log-rank test; relative risk, 2.5 [95% confidence interval, 1.1-5.6]). All STI-positive macaques were SHIV infected, whereas 3 controls (43%) remained uninfected. Moreover, relative to STI-negative animals, SHIV infections occurred earlier in the menstrual cycle in STI-positive macaques (P = .01, by the Wilcoxon test). Levels of inflammatory cytokines (interferon γ, interleukin 6, and granulocyte colony-stimulating factor [G-CSF]) were higher in STI-positive macaques during STI inoculation and SHIV exposure periods (P ≤ .05, by the Wilcoxon test).
Conclusions: C. trachomatis and T. vaginalis infection increase the susceptibility to SHIV, likely because of prolonged genital tract inflammation. These novel data demonstrate a biological link between these nonulcerative STIs and the risk of SHIV infection, supporting epidemiological associations of HIV and STIs. This study establishes a macaque model for studies of high-risk HIV transmission and prevention.
Keywords: Chlamydia; HIV risk; HIV susceptibility model; STI or STD; Trichomonas; macaque; menstrual cycle.
Published by Oxford University Press on behalf of the Infectious Diseases Society of America 2014. This work is written by (a) US Government employee(s) and is in the public domain in the US.
Figures

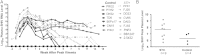

References
-
- Cohen MS. Sexually transmitted diseases enhance HIV transmission: no longer a hypothesis. Lancet. 1998;351(suppl 3):5–7. - PubMed
-
- Gray RH, Wawer MJ, Sewankambo NK, et al. Relative risks and population attributable fraction of incident HIV associated with symptoms of sexually transmitted diseases and treatable symptomatic sexually transmitted diseases in Rakai District, Uganda. Rakai Project Team. AIDS. 1999;13:2113–23. - PubMed
-
- Laga M, Manoka A, Kivuvu M, et al. Non-ulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women: results from a cohort study. AIDS. 1993;7:95–102. - PubMed
-
- Quinn TC. STDs, viral load, circumcision, and the transmission of HIV. In: Braun JF, Horn T, editors. The PRN handbook. New York City: Physicians Research Network; 2000.
-
- Sexton J, Garnett G, Rottingen JA. Metaanalysis and metaregression in interpreting study variability in the impact of sexually transmitted diseases on susceptibility to HIV infection. Sex Transm Dis. 2005;32:351–7. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

