Prenatal alcohol exposure modifies glucocorticoid receptor subcellular distribution in the medial prefrontal cortex and impairs frontal cortex-dependent learning
- PMID: 24755652
- PMCID: PMC3995983
- DOI: 10.1371/journal.pone.0096200
Prenatal alcohol exposure modifies glucocorticoid receptor subcellular distribution in the medial prefrontal cortex and impairs frontal cortex-dependent learning
Abstract
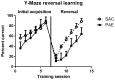
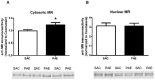
Prenatal alcohol exposure (PAE) has been shown to impair learning, memory and executive functioning in children. Perseveration, or the failure to respond adaptively to changing contingencies, is a hallmark on neurobehavioral assessment tasks for human fetal alcohol spectrum disorder (FASD). Adaptive responding is predominantly a product of the medial prefrontal cortex (mPFC) and is regulated by corticosteroids. In our mouse model of PAE we recently reported deficits in hippocampal formation-dependent learning and memory and a dysregulation of hippocampal formation glucocorticoid receptor (GR) subcellular distribution. Here, we examined the effect of PAE on frontal cortical-dependent behavior, as well as mPFC GR subcellular distribution and the levels of regulators of intracellular GR transport. PAE mice displayed significantly reduced response flexibility in a Y-maze reversal learning task. While the levels of total nuclear GR were reduced in PAE mPFC, levels of GR phosphorylated at serines 203, 211 and 226 were not significantly changed. Cytosolic, but not nuclear, MR levels were elevated in the PAE mPFC. The levels of critical GR trafficking proteins, FKBP51, Hsp90, cyclophilin 40, dynamitin and dynein intermediate chain, were altered in PAE mice, in favor of the exclusion of GR from the nucleus, indicating dysregulation of GR trafficking. Our findings suggest that there may be a link between a deficit in GR nuclear localization and frontal cortical learning deficits in prenatal alcohol-exposed mice.
Conflict of interest statement
Figures




References
-
- Joels M, Sarabdjitsingh RA, Karst H (2012) Unraveling the time domains of corticosteroid hormone influences on brain activity: rapid, slow, and chronic modes. Pharmacological reviews 64: 901–938. - PubMed
-
- Schoneveld OJ, Gaemers IC, Lamers WH (2004) Mechanisms of glucocorticoid signalling. Biochimica et biophysica acta 1680: 114–128. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

