Imaging neural spiking in brain tissue using FRET-opsin protein voltage sensors
- PMID: 24755708
- PMCID: PMC4247277
- DOI: 10.1038/ncomms4674
Imaging neural spiking in brain tissue using FRET-opsin protein voltage sensors
Abstract
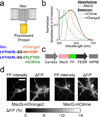
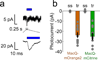
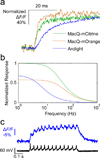
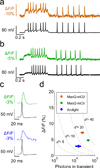
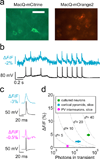
Genetically encoded fluorescence voltage sensors offer the possibility of directly visualizing neural spiking dynamics in cells targeted by their genetic class or connectivity. Sensors of this class have generally suffered performance-limiting tradeoffs between modest brightness, sluggish kinetics and limited signalling dynamic range in response to action potentials. Here we describe sensors that use fluorescence resonance energy transfer (FRET) to combine the rapid kinetics and substantial voltage-dependence of rhodopsin family voltage-sensing domains with the brightness of genetically engineered protein fluorophores. These FRET-opsin sensors significantly improve upon the spike detection fidelity offered by the genetically encoded voltage sensor, Arclight, while offering faster kinetics and higher brightness. Using FRET-opsin sensors we imaged neural spiking and sub-threshold membrane voltage dynamics in cultured neurons and in pyramidal cells within neocortical tissue slices. In live mice, rates and optical waveforms of cerebellar Purkinje neurons' dendritic voltage transients matched expectations for these cells' dendritic spikes.
Figures
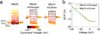

Comment in
-
Visualizing voltage.Nat Methods. 2014 Jul;11(7):710-1. doi: 10.1038/nmeth.3018. Nat Methods. 2014. PMID: 25110781 No abstract available.
References
-
- Grinvald A, Hildesheim R. VSDI: a new era in functional imaging of cortical dynamics. Nat. Rev. Neuro. 2004;5:874–885. - PubMed
-
- Chemla S, Chavane F. Voltage-sensitive dye imaging: Technique review and models. J Physiol Paris. 2010;104:40–50. - PubMed
-
- Salzberg BM, Grinvald A, Cohen LB, Davila HV, Ross WN. Optical Recording of Neuronal Activity in an Invertebrate Central Nervous System: Simultaneous Monitoring of Several Neurons. J. Neurophysiol. 1977;40:1281–1291. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

