Differences in nicotine metabolism of two Nicotiana attenuata herbivores render them differentially susceptible to a common native predator
- PMID: 24755743
- PMCID: PMC3995989
- DOI: 10.1371/journal.pone.0095982
Differences in nicotine metabolism of two Nicotiana attenuata herbivores render them differentially susceptible to a common native predator
Abstract
Background: Nicotiana attenuata is attacked by larvae of both specialist (Manduca sexta) and generalist (Spodoptera exigua) lepidopteran herbivores in its native habitat. Nicotine is one of N. attenuata's important defenses. M. sexta is highly nicotine tolerant; whether cytochrome P450 (CYP)-mediated oxidative detoxification and/or rapid excretion is responsible for its exceptional tolerance remains unknown despite five decades of study. Recently, we demonstrated that M. sexta uses its nicotine-induced CYP6B46 to efflux midgut-nicotine into the hemolymph, facilitating nicotine exhalation that deters predatory wolf spiders (Camptocosa parallela). S. exigua's nicotine metabolism is uninvestigated.
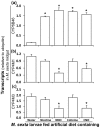
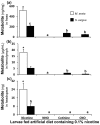
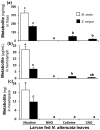
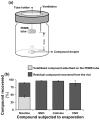
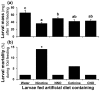
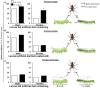
Methodology/principal findings: We compared the ability of these two herbivores to metabolize, tolerate and co-opt ingested nicotine for defense against the wolf spider. In addition, we analyzed the spider's excretion to gain insights into its nicotine metabolism. Contrary to previous reports, we found that M. sexta larvae neither accumulate the common nicotine oxides (cotinine, cotinine N-oxide and nicotine N-oxide) nor excrete them faster than nicotine. In M. sexta larvae, ingestion of nicotine as well as its oxides increases the accumulation of CYP6B46 transcripts. In contrast, S. exigua accumulates nicotine oxides and exhales less (66%) nicotine than does M. sexta. Spiders prefer nicotine-fed S. exigua over M. sexta, a preference reversed by topical or headspace nicotine supplementation, but not ingested or topically-coated nicotine oxides, suggesting that externalized nicotine but not the nicotine detoxification products deter spider predation. The spiders also do not accumulate nicotine oxides.
Conclusions: Nicotine oxidation reduces S. exigua's headspace-nicotine and renders it more susceptible to predation by spiders than M. sexta, which exhales unmetabolized nicotine. These results are consistent with the hypothesis that generalist herbivores incur costs of detoxification, which include the ecological costs of greater predation risks, in addition to the previously demonstrated energetic, physiological and metabolic costs.
Conflict of interest statement
Figures


References
-
- Trigo JR (2011) Effects of pyrrolizidine alkaloids through different trophic levels. Phytochem Rev 10: 83–98.
-
- Cornelius ML, Bernays EA (1995) The effect of plant chemistry on the acceptability of caterpillar prey to the Argentine ant Iridomyrmex humilils (Hymenoptera, Formicidae). J Insect Behav 8: 579–593.
-
- Pentzold S, Zagrobelny M, Rook F, Bak S (2013) How insects overcome two-component plant chemical defence: plant β-glucosidases as the main target for herbivore adaptation. Biol Rev. - PubMed
-
- Pasteels JM, Rowell-Rahier M, Braekman JC, Dupont A (1983) Salicin from host plant as precursor of salicylaldehyde in defensive secretion of Chrysomeline larvae. Physiol Entomol 8: 307–314.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

