Repeated mild traumatic brain injury causes chronic neuroinflammation, changes in hippocampal synaptic plasticity, and associated cognitive deficits
- PMID: 24756076
- PMCID: PMC4083389
- DOI: 10.1038/jcbfm.2014.75
Repeated mild traumatic brain injury causes chronic neuroinflammation, changes in hippocampal synaptic plasticity, and associated cognitive deficits
Abstract
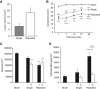
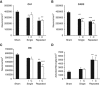
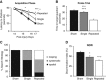
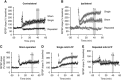
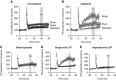
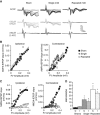
Repeated mild traumatic brain injury (mTBI) can cause sustained cognitive and psychiatric changes, as well as neurodegeneration, but the underlying mechanisms remain unclear. We examined histologic, neurophysiological, and cognitive changes after single or repeated (three injuries) mTBI using the rat lateral fluid percussion (LFP) model. Repeated mTBI caused substantial neuronal cell loss and significantly increased numbers of activated microglia in both ipsilateral and contralateral hippocampus on post-injury day (PID) 28. Long-term potentiation (LTP) could not be induced on PID 28 after repeated mTBI in ex vivo hippocampal slices from either hemisphere. N-Methyl-D-aspartate (NMDA) receptor-mediated responses were significantly attenuated after repeated mTBI, with no significant changes in α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor-mediated responses. Long-term potentiation was elicited in slices after single mTBI, with potentiation significantly increased in ipsilateral versus contralateral hippocampus. After repeated mTBI, rats displayed cognitive impairments in the Morris water maze (MWM) and novel object recognition (NOR) tests. Thus, repeated mTBI causes deficits in the hippocampal function and changes in excitatory synaptic neurotransmission, which are associated with chronic neuroinflammation and neurodegeneration.
Figures
References
-
- Report to Congress on mild traumatic brain injury in the United States: steps to prevent a serious public health problem . Centers for Disease Control and Prevention (CDC) National Center for Injury Prevention and Control: Atlanta (GA); 2003.
-
- Omalu B, Hammers JL, Bailes J, Hamilton RL, Kamboh MI, Webster G, et al. Chronic traumatic encephalopathy in an Iraqi war veteran with posttraumatic stress disorder who committed suicide. Neurosurg Focus. 2011;31:E3. - PubMed
-
- Omalu B, Bailes J, Hamilton RL, Kamboh MI, Hammers J, Case M, et al. Emerging histomorphologic phenotypes of chronic traumatic encephalopathy in American athletes Neurosurgery 201169173–183.discussion 183. - PubMed
-
- Shultz SR, Bao F, Omana V, Chiu C, Brown A, Cain DP. Repeated mild lateral fluid percussion brain injury in the rat causes cumulative long-term behavioral impairments, neuroinflammation, and cortical loss in an animal model of repeated concussion. J Neurotrauma. 2012;29:281–294. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

