Self-assembly of peptides to nanostructures
- PMID: 24756480
- PMCID: PMC4038164
- DOI: 10.1039/c4ob00447g
Self-assembly of peptides to nanostructures
Abstract
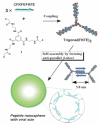
The formation of well-ordered nanostructures through self-assembly of diverse organic and inorganic building blocks has drawn much attention owing to their potential applications in biology and chemistry. Among all organic building blocks, peptides are one of the most promising platforms due to their biocompatibility, chemical diversity, and resemblance to proteins. Inspired by the protein assembly in biological systems, various self-assembled peptide structures have been constructed using several amino acids and sequences. This review focuses on this emerging area, the recent advances in peptide self-assembly, and formation of different nanostructures, such as tubular structures, fibers, vesicles, and spherical and rod-coil structures. While different peptide nanostructures have been discovered, potential applications are explored in drug delivery, tissue engineering, wound healing, and surfactants.
Figures







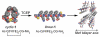













References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

