Molecular mechanisms of neuronal nitric oxide synthase in cardiac function and pathophysiology
- PMID: 24756636
- PMCID: PMC4146369
- DOI: 10.1113/jphysiol.2013.270306
Molecular mechanisms of neuronal nitric oxide synthase in cardiac function and pathophysiology
Abstract
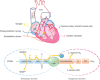
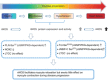
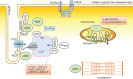
Neuronal nitric oxide synthase (nNOS or NOS1) is the major endogenous source of myocardial nitric oxide (NO), which facilitates cardiac relaxation and modulates contraction. In the healthy heart it regulates intracellular Ca(2+), signalling pathways and oxidative homeostasis and is upregulated from early phases upon pathogenic insult. nNOS plays pivotal roles in protecting the myocardium from increased oxidative stress, systolic/diastolic dysfunction, adverse structural remodelling and arrhythmias in the failing heart. Here, we show that the downstream target proteins of nNOS and underlying post-transcriptional modifications are shifted during disease progression from Ca(2+)-handling proteins [e.g. PKA-dependent phospholamban phosphorylation (PLN-Ser(16))] in the healthy heart to cGMP/PKG-dependent PLN-Ser(16) with acute angiotensin II (Ang II) treatment. In early hypertension, nNOS-derived NO is involved in increases of cGMP/PKG-dependent troponin I (TnI-Ser(23/24)) and cardiac myosin binding protein C (cMBP-C-Ser(273)). However, nNOS-derived NO is shown to increase S-nitrosylation of various Ca(2+)-handling proteins in failing myocardium. The spatial compartmentation of nNOS and its translocation for diverse binding partners in the diseased heart or various nNOS splicing variants and regulation in response to pathological stress may be responsible for varied underlying mechanisms and functions. In this review, we endeavour to outline recent advances in knowledge of the molecular mechanisms mediating the functions of nNOS in the myocardium in both normal and diseased hearts. Insights into nNOS gene regulation in various tissues are discussed. Overall, nNOS is an important cardiac protector in the diseased heart. The dynamic localization and various mediating mechanisms of nNOS ensure that it is able to regulate functions effectively in the heart under stress.
© 2014 The Authors. The Journal of Physiology © 2014 The Physiological Society.
Figures

Comment in
-
Cross-talking. Ca2+, H+ and nitric oxide.J Physiol. 2014 Aug 1;592(15):3177-8. doi: 10.1113/jphysiol.2014.278697. J Physiol. 2014. PMID: 25085973 Free PMC article. No abstract available.
References
-
- Adachi T, Weisbrod RM, Pimentel DR, Ying J, Sharov VS, Schöneich C, Cohen RA. S-glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat Med. 2004;10:1200–1207. - PubMed
-
- Aragón JP, Condit ME, Bhushan S, Predmore BL, Patel SS, Grinsfelder DB, Gundewar S, Jha S, Calvert JW, Barouch LA, Lavu M, Wright HM, Lefer DJ. β3-adrenoreceptor stimulation ameliorates myocardial ischemia–reperfusion injury via endothelial nitric oxide synthase and neuronal nitric oxide synthase activation. J Am Coll Cardiol. 2011;58:2683–2691. - PMC - PubMed
-
- Arking DE, Pfeufer A, Post W, Kao WH, Newton-Cheh C, Ikeda M, West K, Kashuk C, Akyol M, Perz S, Jalilzadeh S, Illig T, Gieger C, Guo CY, Larson MG, Wichmann HE, Marbán E, O'Donnell CJ, Hirschhorn JN, Kääb S, Spooner PM, Meitinger T, Chakravarti A. A common genetic variant in the NOS1 regulator NOS1AP modulates cardiac repolarization. Nat Genet. 2006;38:644–651. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

