A novel short-term plasticity of intrinsic excitability in the hippocampal CA1 pyramidal cells
- PMID: 24756640
- PMCID: PMC4221824
- DOI: 10.1113/jphysiol.2014.273185
A novel short-term plasticity of intrinsic excitability in the hippocampal CA1 pyramidal cells
Abstract
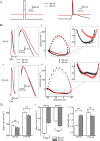
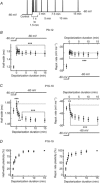
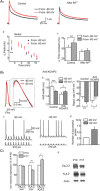
Changes in neuronal activity often trigger compensatory mechanisms aimed at regulating network activity homeostatically. Here we have identified and characterized a novel form of compensatory short-term plasticity of membrane excitability, which develops early after the eye-opening period in rats (P16-19 days) but not before that developmental stage (P9-12 days old). Holding the membrane potential of CA1 neurons right below the firing threshold from 15 s to several minutes induced a potentiation of the repolarizing phase of the action potentials that contributed to a decrease in the firing rate of CA1 pyramidal neurons in vitro. Furthermore, the mechanism for inducing this plasticity required the action of intracellular Ca(2+) entering through T-type Ca(2+) channels. This increase in Ca(2+) subsequently activated the Ca(2+) sensor K(+) channel interacting protein 3, which led to the increase of an A-type K(+) current. These results suggest that Ca(2+) modulation of somatic A-current represents a new form of homeostatic regulation that provides CA1 pyramidal neurons with the ability to preserve their firing abilities in response to membrane potential variations on a scale from tens of seconds to several minutes.
© 2014 The Authors. The Journal of Physiology © 2014 The Physiological Society.
Figures






References
-
- Aizenman CD, Linden DJ. Rapid, synaptically driven increases in the intrinsic excitability of cerebellar deep nuclear neurons. Nat Neurosci. 2000;3:109–111. - PubMed
-
- Anderson D, Mehaffey WH, Iftinca M, Rehak R, Engbers JD, Hameed S, Zamponi GW, Turner RW. Regulation of neuronal activity by Cav3-Kv4 channel signaling complexes. Nat Neurosci. 2010;13:333–337. - PubMed
-
- Blanton MG, Lo Turco JJ, Kriegstein AR. Whole cell recording from neurons in slices of reptilian and mammalian cerebral cortex. J Neurosci Methods. 1989;30:203–210. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

