Geldanamycin attenuates 3‑nitropropionic acid‑induced apoptosis and JNK activation through the expression of HSP 70 in striatal cells
- PMID: 24756698
- PMCID: PMC4072345
- DOI: 10.3892/ijmm.2014.1747
Geldanamycin attenuates 3‑nitropropionic acid‑induced apoptosis and JNK activation through the expression of HSP 70 in striatal cells
Abstract
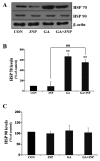
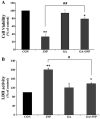
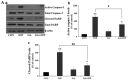
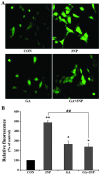
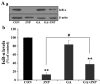
Although selective striatal cell death is a characteristic hallmark in the pathogenesis of Huntington's disease (HD), the underlying mechanism of striatal susceptibility remains to be clarified. Heat shock proteins (HSPs) have been reported to suppress the aggregate formation of mutant huntingtin and concurrent striatal cell death. In a previous study, we observed that heat shock transcription factor 1 (HSF1), a major transcription factor of HSPs, significantly attenuated 3‑nitropropionic acid (3NP)‑induced reactive oxygen species (ROS) production and apoptosis through the expression of HSP 70 in striatal cells. To investigate the differential roles of HSPs in 3NP‑induced striatal cell death, the effect of geldanamycin (GA), an HSP 90 inhibitor, was examined in 3NP‑stimulated striatal cells. GA significantly attenuated 3NP‑induced striatal apoptosis and ROS production with an increased expression of HSP 70. Triptolide (TL), an HSP 70 inhibitor, abolished GA‑mediated protective effects in 3NP‑stimulated striatal cells. To understand the underlying mechanism by which GA‑mediated HSP 70 protects striatal cells against 3NP stimulation, the involvement of various signaling pathways was examined. GA significantly attenuated 3NP‑induced c‑Jun N‑terminal kinase (JNK) phosphorylation and subsequent c‑Jun phosphorylation in striatal cells. Taken together, the present study demonstrated that GA exhibits protective properties against 3NP‑induced apoptosis and JNK activation via the induction of HSP 70 in striatal cells, suggesting that expression of HSP 70 may be a valuable therapeutic target in the treatment of HD.
Figures






References
-
- Andrew SE, Goldberg YP, Kremer B, et al. The relationship between trinucleotide (CAG) repeat length and clinical features of Huntington’s disease. Nat Genet. 1993;4:398–403. - PubMed
-
- Vonsattel JP, DiFiglia M. Huntington disease. J Neuropathol Exp Neurol. 1998;57:369–384. - PubMed
-
- Beal MF. Mitochondria take center stage in aging and neurodegeneration. Ann Neurol. 2005;58:495–505. - PubMed
-
- Oliveira JM, Jekabsons MB, Chen S, et al. Mitochondrial dysfunction in Huntington’s disease: the bioenergetics of isolated and in situ mitochondria from transgenic mice. J Neurochem. 2007;101:241–249. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

