Neocortical Tet3-mediated accumulation of 5-hydroxymethylcytosine promotes rapid behavioral adaptation
- PMID: 24757058
- PMCID: PMC4024925
- DOI: 10.1073/pnas.1318906111
Neocortical Tet3-mediated accumulation of 5-hydroxymethylcytosine promotes rapid behavioral adaptation
Abstract
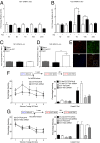
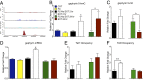
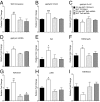
5-hydroxymethylcytosine (5-hmC) is a novel DNA modification that is highly enriched in the adult brain and dynamically regulated by neural activity. 5-hmC accumulates across the lifespan; however, the functional relevance of this change in 5-hmC and whether it is necessary for behavioral adaptation have not been fully elucidated. Moreover, although the ten-eleven translocation (Tet) family of enzymes is known to be essential for converting methylated DNA to 5-hmC, the role of individual Tet proteins in the adult cortex remains unclear. Using 5-hmC capture together with high-throughput DNA sequencing on individual mice, we show that fear extinction, an important form of reversal learning, leads to a dramatic genome-wide redistribution of 5-hmC within the infralimbic prefrontal cortex. Moreover, extinction learning-induced Tet3-mediated accumulation of 5-hmC is associated with the establishment of epigenetic states that promote gene expression and rapid behavioral adaptation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

