The neurobiological basis of cognitive impairment in Parkinson's disease
- PMID: 24757112
- PMCID: PMC4049032
- DOI: 10.1002/mds.25857
The neurobiological basis of cognitive impairment in Parkinson's disease
Abstract
The recent formalization of clinical criteria for Parkinson's disease with dementia (PDD) codifies many studies on this topic, including those assessing biological correlates. These studies show that the emergence of PDD occurs on the background of severe dopamine deficits with, the main pathological drivers of cognitive decline being a synergistic effect between alpha-synuclein and Alzheimer's disease pathology. The presence of these pathologies correlates with a marked loss of limbic and cortically projecting dopamine, noradrenaline, serotonin, and acetylcholine neurons, although the exact timing of these relationships remains to be determined. Genetic factors, such as triplications in the α-synuclein gene, lead to a clear increased risk of PDD, whereas others, such as parkin mutations, are associated with a reduced risk of PDD. The very recent formalization of clinical criteria for PD with mild cognitive impairment (PD-MCI) allows only speculation on its biological and genetic bases. Critical assessment of animal models shows that chronic low-dose MPTP treatment in primates recapitulates PD-MCI over time, enhancing the current biological concept of PD-MCI as having enhanced dopamine deficiency in frontostriatal pathways as well as involvement of other neurotransmitter systems. Data from other animal models support multiple transmitter involvement in cognitive impairment in PD. Whereas dopamine dysfunction has been highlighted because of its obvious role in PD, the role of the other neurotransmitter systems, neurodegenerative pathologies, and genetic factors in PD-MCI remains to be fully elucidated.
Keywords: Parkinson's disease dementia; genetic risk; neuropathology; neurotransmitters; preclinical models.
© 2014 International Parkinson and Movement Disorder Society.
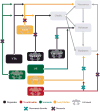
Figures





References
-
- Dickson DW, Braak H, Duda JE, et al. Neuropathological assessment of Parkinson's disease: refining the diagnostic criteria. Lancet Neurol. 2009;8(12):1150–1157. - PubMed
-
- Halliday GM, McCann H. The progression of pathology in Parkinson's disease. Ann N Y Acad Sci. 2010;1184:188–195. - PubMed
-
- Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord. 2007;22(12):1689–1707. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

