Overnight closed-loop insulin delivery in young people with type 1 diabetes: a free-living, randomized clinical trial
- PMID: 24757227
- PMCID: PMC3994941
- DOI: 10.2337/dc13-2644
Overnight closed-loop insulin delivery in young people with type 1 diabetes: a free-living, randomized clinical trial
Abstract
Objective: To evaluate feasibility, safety, and efficacy of overnight closed-loop insulin delivery in free-living youth with type 1 diabetes.
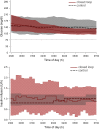
Research design and methods: Overnight closed loop was evaluated at home by 16 pump-treated adolescents with type 1 diabetes aged 12-18 years. Over a 3-week period, overnight insulin delivery was directed by a closed-loop system, and on another 3-week period sensor-augmented therapy was applied. The order of interventions was random. The primary end point was time when adjusted sensor glucose was between 3.9 and 8.0 mmol/L from 2300 to 0700 h.
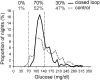
Results: Closed loop was constantly applied over at least 4 h on 269 nights (80%); sensor data were collected over at least 4 h on 282 control nights (84%). Closed loop increased time spent with glucose in target by a median 15% (interquartile range -9 to 43; P < 0.001). Mean overnight glucose was reduced by a mean 14 (SD 58) mg/dL (P < 0.001). Time when glucose was <70 mg/dL was low in both groups, but nights with glucose <63 mg/dL for at least 20 min were less frequent during closed loop (10 vs. 17%; P = 0.01). Despite lower total daily insulin doses by a median 2.3 (interquartile range -4.7 to 9.3) units (P = 0.009), overall 24-h glucose was reduced by a mean 9 (SD 41) mg/dL (P = 0.006) during closed loop.
Conclusions: Unsupervised home use of overnight closed loop in adolescents with type 1 diabetes is safe and feasible. Glucose control was improved during the day and night with fewer episodes of nocturnal hypoglycemia.
Trial registration: ClinicalTrials.gov NCT01221467.
Figures

References
-
- Matyka KA, Crowne EC, Havel PJ, Macdonald IA, Matthews D, Dunger DB. Counterregulation during spontaneous nocturnal hypoglycemia in prepubertal children with type 1 diabetes. Diabetes Care 1999;22:1144–1150 - PubMed
-
- Wood JR, Miller KM, Maahs DM, et al. T1D Exchange Clinic Network Most youth with type 1 diabetes in the T1D Exchange Clinic Registry do not meet American Diabetes Association or International Society for Pediatric and Adolescent Diabetes clinical guidelines. Diabetes Care 2013;36:2035–2037 - PMC - PubMed
-
- Pickup JC. Insulin-pump therapy for type 1 diabetes mellitus. N Engl J Med 2012;366:1616–1624 - PubMed
-
- Phillip M, Danne T, Shalitin S, et al. Consensus Forum Participants Use of continuous glucose monitoring in children and adolescents. Pediatr Diabetes 2012;13:215–228 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

