5-azacytidine promotes an inhibitory T-cell phenotype and impairs immune mediated antileukemic activity
- PMID: 24757283
- PMCID: PMC3976863
- DOI: 10.1155/2014/418292
5-azacytidine promotes an inhibitory T-cell phenotype and impairs immune mediated antileukemic activity
Abstract
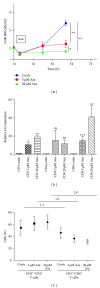
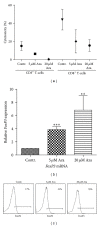
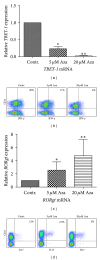
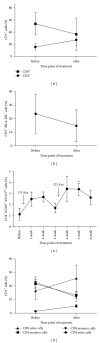
Demethylating agent, 5-Azacytidine (5-Aza), has been shown to be active in treatment of myeloid malignancies. 5-Aza enhances anticancer immunity, by increasing expression of tumor-associated antigens. However, the impact of 5-Aza immune responses remains poorly understood. Here, T-cell mediated tumor immunity effects of 5-Aza, are investigated in vitro and in vivo. T-cells from healthy donors were treated with 5-Aza and analyzed by qRT-PCR and flow cytometry for changes in gene expression and phenotype. Functionality was assessed by a tumor lysis assay. Peripheral blood from patients treated with 5-Aza after alloSCT was monitored for changes in T-cell subpopulations. 5-Aza treatment resulted in a decrease in CD8+ T-cells, whereas CD4+ T-cells increased. Furthermore, numbers of IFN-γ + T-helper 1 cells (Th1) were reduced, while Treg-cells showed substantial increase. Additionally, CD8+ T-cells exhibited limited killing capacity against leukemic target cells. In vivo data confirm the increase of Treg compartment, while CD8+ T-effector cell numbers were reduced. 5-Aza treatment results in a shift from cytotoxic to regulatory T-cells with a functional phenotype and a major reduction in proinflammatory Th1-cells, indicating a strong inhibition of tumor-specific T-cell immunity by 5-Aza.
Figures



Comment in
-
Comment on "5-azacytidine promotes an inhibitory T-cell phenotype and impairs immune mediated antileukemic activity".Mediators Inflamm. 2015;2015:871641. doi: 10.1155/2015/871641. Epub 2015 Mar 8. Mediators Inflamm. 2015. PMID: 25838640 Free PMC article. No abstract available.
References
-
- Delcuve GP, Rastegar M, Davie JR. Epigenetic control. Journal of Cellular Physiology. 2009;219(2):243–250. - PubMed
-
- Esteller M. Cancer epigenomics: DNA methylomes and histone-modification maps. Nature Reviews Genetics. 2007;8(4):286–298. - PubMed
-
- Silverman LR, Demakos EP, Peterson BL, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. Journal of Clinical Oncology. 2002;20(10):2429–2440. - PubMed
-
- Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. Journal of Clinical Oncology. 2010;28(4):562–569. - PubMed
-
- Stresemann C, Lyko F. Modes of action of the DNA methyltransferase inhibitors azacytidine and decitabine. International Journal of Cancer. 2008;123(1):8–13. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

