Fluoxetine dose and administration method differentially affect hippocampal plasticity in adult female rats
- PMID: 24757568
- PMCID: PMC3976918
- DOI: 10.1155/2014/123026
Fluoxetine dose and administration method differentially affect hippocampal plasticity in adult female rats
Abstract
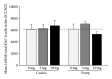
Selective serotonin reuptake inhibitor medications are one of the most common treatments for mood disorders. In humans, these medications are taken orally, usually once per day. Unfortunately, administration of antidepressant medications in rodent models is often through injection, oral gavage, or minipump implant, all relatively stressful procedures. The aim of the present study was to investigate how administration of the commonly used SSRI, fluoxetine, via a wafer cookie, compares to fluoxetine administration using an osmotic minipump, with regards to serum drug levels and hippocampal plasticity. For this experiment, adult female Sprague-Dawley rats were divided over the two administration methods: (1) cookie and (2) osmotic minipump and three fluoxetine treatment doses: 0, 5, or 10 mg/kg/day. Results show that a fluoxetine dose of 5 mg/kg/day, but not 10 mg/kg/day, results in comparable serum levels of fluoxetine and its active metabolite norfluoxetine between the two administration methods. Furthermore, minipump administration of fluoxetine resulted in higher levels of cell proliferation in the granule cell layer (GCL) at a 5 mg dose compared to a 10 mg dose. Synaptophysin expression in the GCL, but not CA3, was significantly lower after fluoxetine treatment, regardless of administration method. These data suggest that the administration method and dose of fluoxetine can differentially affect hippocampal plasticity in the adult female rat.
Figures



Similar articles
-
Developmental exposure to SSRIs, in addition to maternal stress, has long-term sex-dependent effects on hippocampal plasticity.Psychopharmacology (Berl). 2015 Apr;232(7):1231-44. doi: 10.1007/s00213-014-3758-0. Epub 2014 Oct 12. Psychopharmacology (Berl). 2015. PMID: 25304865
-
Gestational stress and fluoxetine treatment differentially affect plasticity, methylation and serotonin levels in the PFC and hippocampus of rat dams.Neuroscience. 2016 Jul 7;327:32-43. doi: 10.1016/j.neuroscience.2016.03.068. Epub 2016 Apr 11. Neuroscience. 2016. PMID: 27060483
-
Fluoxetine selectively alters 5-hydroxytryptamine1A and gamma-aminobutyric acidB receptor-mediated hyperpolarization in area CA1, but not area CA3, hippocampal pyramidal cells.J Pharmacol Exp Ther. 1997 Apr;281(1):115-22. J Pharmacol Exp Ther. 1997. PMID: 9103487
-
Perinatal fluoxetine effects on social play, the HPA system, and hippocampal plasticity in pre-adolescent male and female rats: Interactions with pre-gestational maternal stress.Psychoneuroendocrinology. 2017 Oct;84:159-171. doi: 10.1016/j.psyneuen.2017.07.480. Epub 2017 Jul 15. Psychoneuroendocrinology. 2017. PMID: 28735226
-
Pharmacokinetics of fluoxetine in rhesus macaques following multiple routes of administration.Pharmacology. 2011;88(1-2):44-9. doi: 10.1159/000329417. Epub 2011 Jul 14. Pharmacology. 2011. PMID: 21757974 Free PMC article.
Cited by
-
Antidepressant-like Activity of Patchouli Oil var. Tapak Tuan (Pogostemon cablin Benth) via Elevated Dopamine Level: A Study Using Rat Model.Pharmaceuticals (Basel). 2022 May 15;15(5):608. doi: 10.3390/ph15050608. Pharmaceuticals (Basel). 2022. PMID: 35631434 Free PMC article.
-
Chronic stress prior to pregnancy potentiated long-lasting postpartum depressive-like behavior, regulated by Akt-mTOR signaling in the hippocampus.Sci Rep. 2016 Oct 19;6:35042. doi: 10.1038/srep35042. Sci Rep. 2016. PMID: 27756905 Free PMC article.
-
The Efficacy of N-Acetyl-Cysteine (NAC) Supplementation in FST Model for Screening Antidepressants.Basic Clin Neurosci. 2022 Nov-Dec;13(6):839-854. doi: 10.32598/bcn.2023.2356.2. Epub 2022 Nov 1. Basic Clin Neurosci. 2022. PMID: 37323955 Free PMC article.
-
Developmental changes in serotonin signaling: Implications for early brain function, behavior and adaptation.Neuroscience. 2017 Feb 7;342:212-231. doi: 10.1016/j.neuroscience.2016.02.037. Epub 2016 Feb 22. Neuroscience. 2017. PMID: 26905950 Free PMC article. Review.
-
The tricyclic antidepressant clomipramine inhibits neuronal autophagic flux.Sci Rep. 2019 Mar 19;9(1):4881. doi: 10.1038/s41598-019-40887-x. Sci Rep. 2019. PMID: 30890728 Free PMC article.
References
-
- Lucassen PJ, Meerlo P, Naylor AS, et al. Regulation of adult neurogenesis by stress, sleep disruption, exercise and inflammation: implications for depression and antidepressant action. European Neuropsychopharmacology. 2010;20(1):1–17. - PubMed
-
- Santarelli L, Saxe M, Gross C, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301(5634):805–809. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

