Absorbable hydrogel spacer use in men undergoing prostate cancer radiotherapy: 12 month toxicity and proctoscopy results of a prospective multicenter phase II trial
- PMID: 24758224
- PMCID: PMC4016630
- DOI: 10.1186/1748-717X-9-96
Absorbable hydrogel spacer use in men undergoing prostate cancer radiotherapy: 12 month toxicity and proctoscopy results of a prospective multicenter phase II trial
Abstract
Background: Radiation therapy is one of the recommended treatment options for localized prostate cancer. In randomized trials, dose escalation was correlated with better biochemical control but also with higher rectal toxicity. A prospective multicenter phase II study was carried out to evaluate the safety, clinical and dosimetric effects of the hydrogel prostate-rectum spacer. Here we present the 12 months toxicity results of this trial.
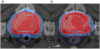
Methods: Fifty two patients with localized prostate cancer received a transperineal PEG hydrogel injection between the prostate and rectum, and then received IMRT to a dose of 78 Gy. Gastrointestinal and genitourinary toxicity were recorded during treatment and at 3, 6 and 12 months following irradiation by using the RTOG/EORTC criteria. Additionally, proctoscopy was performed 12 months after treatment and the results were scored using the Vienna Rectoscopy Scale (VRS).
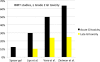
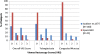
Results: Of the patients treated 39.6% and 12.5% experienced acute Grade 1 and Grade 2 GI toxicity, respectively. There was no Grade 3 or Grade 4 acute GI toxicity experienced in the study. Only 4.3% showed late Grade 1 GI toxicity, and there was no late Grade 2 or greater GI toxicity experienced in the study. A total of 41.7%, 35.4% and 2.1% of the men experienced acute Grade 1, Grade 2 and Grade 3 GU toxicity, respectively. There was no Grade 4 acute GU toxicity experienced in the study. Late Grade 1 and Grade 2 GU toxicity was experienced in 17.0% and 2.1% of the patients, respectively. There was no late Grade 3 or greater GU toxicity experienced in the study. Seventy one percent of the patients had a VRS score of 0, and one patient (2%) had Grade 3 teleangiectasia. There was no evidence of ulceration, stricture or necrosis at 12 months.
Conclusion: The use of PEG spacer gel is a safe and effective method to spare the rectum from higher dose and toxicity.
Figures
References
-
- Heidenreich A, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V, Mottet N, Schmid HP, van der Kwast T, Wiegel T, Zattoni F. European Association of Urology. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol. 2011;59:61–71. doi: 10.1016/j.eururo.2010.10.039. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

