Targeting the Achilles heel of multidrug-resistant cancer by exploiting the fitness cost of resistance
- PMID: 24758331
- PMCID: PMC4059772
- DOI: 10.1021/cr4006236
Targeting the Achilles heel of multidrug-resistant cancer by exploiting the fitness cost of resistance
Figures



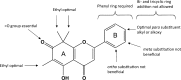
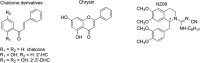
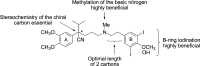
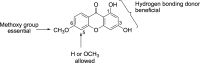
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

