Bone morphogenetic proteins and their antagonists: current and emerging clinical uses
- PMID: 24758361
- PMCID: PMC4128061
- DOI: 10.1111/bph.12724
Bone morphogenetic proteins and their antagonists: current and emerging clinical uses
Abstract
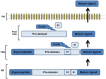
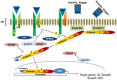
Bone morphogenetic proteins (BMPs) are members of the TGFβ superfamily of secreted cysteine knot proteins that includes TGFβ1, nodal, activins and inhibins. BMPs were first discovered by Urist in the 1960s when he showed that implantation of demineralized bone into intramuscular tissue of rabbits induced bone and cartilage formation. Since this seminal discovery, BMPs have also been shown to play key roles in several other biological processes, including limb, kidney, skin, hair and neuronal development, as well as maintaining vascular homeostasis. The multifunctional effects of BMPs make them attractive targets for the treatment of several pathologies, including bone disorders, kidney and lung fibrosis, and cancer. This review will summarize current knowledge on the BMP signalling pathway and critically evaluate the potential of recombinant BMPs as pharmacological agents for the treatment of bone repair and tissue fibrosis in patients.
Keywords: bone morphogenetic protein; fibrosis; fracture; gremlin; inhibitors; scarring; skeleton.
© 2014 The British Pharmacological Society.
Figures
References
-
- Aoki H, Fujii M, Imamura T, Yagi K, Takehara K, Kato M, et al. Synergistic effects of different bone morphogenetic protein type I receptors on alkaline phosphatase induction. J Cell Sci. 2001;114((Pt 8)):1483–1489. - PubMed
-
- Balemans W, Van Hul W. Extracellular regulation of BMP signaling in vertebrates: a cocktail of modulators. Dev Biol. 2002;250:231–250. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

