Evaluation of multiple-turnover capability of locked nucleic acid antisense oligonucleotides in cell-free RNase H-mediated antisense reaction and in mice
- PMID: 24758560
- PMCID: PMC4106378
- DOI: 10.1089/nat.2013.0470
Evaluation of multiple-turnover capability of locked nucleic acid antisense oligonucleotides in cell-free RNase H-mediated antisense reaction and in mice
Abstract
The multiple-turnover ability of a series of locked nucleic acid (LNA)-based antisense oligonucleotides (AONs) in the RNase H-mediated scission reaction was estimated using a newly developed cell-free reaction system. We determined the initial reaction rates of AONs under multiple-turnover conditions and found that among 24 AONs tested, AONs with melting temperatures (Tm) of 40°C-60°C efficiently elicit multiple rounds of RNA scission. On the other hand, by measuring Tm with two 10-mer RNAs partially complementary to AONs as models of cleaved 5' and 3' fragments of mRNA, we found that AONs require adequate binding affinity for efficient turnover activities. We further demonstrated that the efficacy of a set of 13-mer AONs in mice correlated with their turnover efficiency, indicating that the intracellular situation where AONs function is similar to multiple-turnover conditions. Our methodology and findings may provide an opportunity to shed light on a previously unknown antisense mechanism, leading to further improvement of the activity and safety profiles of AONs.
Figures
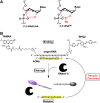
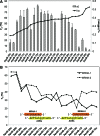
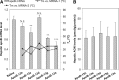
References
-
- CROOKE T.S. (2007). Antisense Drug Technologies: Principles, Strategies, and Applications. 2nd ed. (CRC Press Taylor & Francis Group, Boca Raton, FL: )
-
- GAO W.Y., HAN F.S., STORM C., EGAN W., and CHENG Y.C. (1992). Phosphorothioate oligonucleotides are inhibitors of human DNA polymerases and RNase H: implications for antisense technology. Mol. Pharmacol. 41,223–229 - PubMed
-
- HARI Y., OBIKA S., OHNISHI R., EGUCHI K., OSAKI T., OHISHI H., and IMANISHI T. (2006). Synthesis and properties of 2′-O,4-C-methyleneoxymethylene bridged nucleic acid. Bioorgan. Med. Chem. 14,1029–1038 - PubMed
-
- HUTVAGNER G., and ZAMORE P.D. (2002). A microRNA in a multiple-turnover RNAi enzyme complex. Science 297,2056–2060 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

