Mechanisms of activation of receptor tyrosine kinases: monomers or dimers
- PMID: 24758840
- PMCID: PMC4092861
- DOI: 10.3390/cells3020304
Mechanisms of activation of receptor tyrosine kinases: monomers or dimers
Abstract
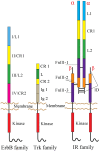
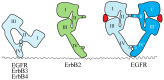
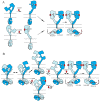
Receptor tyrosine kinases (RTKs) play essential roles in cellular processes, including metabolism, cell-cycle control, survival, proliferation, motility and differentiation. RTKs are all synthesized as single-pass transmembrane proteins and bind polypeptide ligands, mainly growth factors. It has long been thought that all RTKs, except for the insulin receptor (IR) family, are activated by ligand-induced dimerization of the receptors. An increasing number of diverse studies, however, indicate that RTKs, previously thought to exist as monomers, are present as pre-formed, yet inactive, dimers prior to ligand binding. The non-covalently associated dimeric structures are reminiscent of those of the IR family, which has a disulfide-linked dimeric structure. Furthermore, recent progress in structural studies has provided insight into the underpinnings of conformational changes during the activation of RTKs. In this review, I discuss two mutually exclusive models for the mechanisms of activation of the epidermal growth factor receptor, the neurotrophin receptor and IR families, based on these new insights.
Figures
Similar articles
-
Activation of the EGF Receptor by Ligand Binding and Oncogenic Mutations: The "Rotation Model".Cells. 2017 Jun 2;6(2):13. doi: 10.3390/cells6020013. Cells. 2017. PMID: 28574446 Free PMC article. Review.
-
Receptor tyrosine kinases: mechanisms of activation and signaling.Curr Opin Cell Biol. 2007 Apr;19(2):117-23. doi: 10.1016/j.ceb.2007.02.010. Epub 2007 Feb 16. Curr Opin Cell Biol. 2007. PMID: 17306972 Free PMC article. Review.
-
Structural analysis of receptor tyrosine kinases.Prog Biophys Mol Biol. 1999;71(3-4):343-58. doi: 10.1016/s0079-6107(98)00047-9. Prog Biophys Mol Biol. 1999. PMID: 10354703 Review.
-
Insulin and epidermal growth factor receptor family members share parallel activation mechanisms.Protein Sci. 2020 Jun;29(6):1331-1344. doi: 10.1002/pro.3871. Epub 2020 Apr 28. Protein Sci. 2020. PMID: 32297376 Free PMC article. Review.
-
Dimerization of the Trk receptors in the plasma membrane: effects of their cognate ligands.Biochem J. 2018 Nov 30;475(22):3669-3685. doi: 10.1042/BCJ20180637. Biochem J. 2018. PMID: 30366959 Free PMC article.
Cited by
-
Recent developments in receptor tyrosine kinases targeted anticancer therapy.Vet World. 2016 Jan;9(1):80-90. doi: 10.14202/vetworld.2016.80-90. Epub 2016 Jan 29. Vet World. 2016. PMID: 27051190 Free PMC article. Review.
-
Interferon-induced transmembrane protein 1-mediated EGFR/SOX2 signaling axis is essential for progression of non-small cell lung cancer.Int J Cancer. 2019 Apr 15;144(8):2020-2032. doi: 10.1002/ijc.31926. Epub 2018 Dec 8. Int J Cancer. 2019. PMID: 30318841 Free PMC article.
-
Visualization of ligand-induced transmembrane signaling in the full-length human insulin receptor.J Cell Biol. 2018 May 7;217(5):1643-1649. doi: 10.1083/jcb.201711047. Epub 2018 Feb 16. J Cell Biol. 2018. PMID: 29453311 Free PMC article.
-
A Generalizable Optogenetic Strategy to Regulate Receptor Tyrosine Kinases during Vertebrate Embryonic Development.J Mol Biol. 2020 May 1;432(10):3149-3158. doi: 10.1016/j.jmb.2020.03.032. Epub 2020 Apr 8. J Mol Biol. 2020. PMID: 32277988 Free PMC article.
-
Tyrosine phosphatase PTPN11/SHP2 in solid tumors - bull's eye for targeted therapy?Front Immunol. 2024 Mar 5;15:1340726. doi: 10.3389/fimmu.2024.1340726. eCollection 2024. Front Immunol. 2024. PMID: 38504984 Free PMC article. Review.
References
-
- Gotoh N., Tojo A., Hino M., Yazaki Y., Shibuya M. A highly conserved tyrosine residue at codon 845 within the kinase domain is not required for the transforming activity of human epidermal growth factor receptor. Biochem. Biophys. Res. Commun. 1992;186:768–774. doi: 10.1016/0006-291X(92)90812-Y. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

