RutheniumII complexes bearing fused polycyclic ligands: from fundamental aspects to potential applications
- PMID: 24759069
- PMCID: PMC6270827
- DOI: 10.3390/molecules19045028
RutheniumII complexes bearing fused polycyclic ligands: from fundamental aspects to potential applications
Abstract
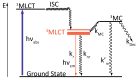
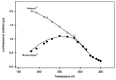
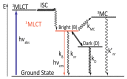
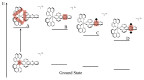
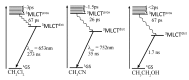
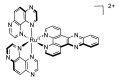
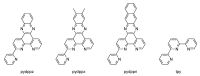
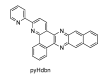
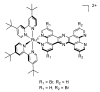
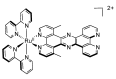
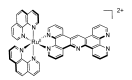
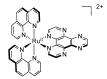
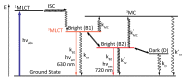
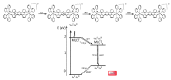
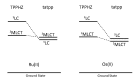
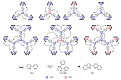
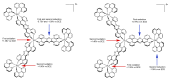
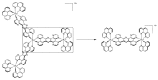
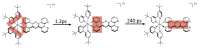
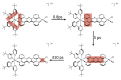
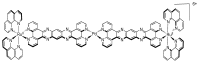
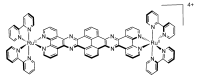
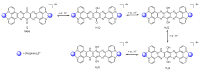
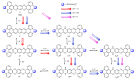
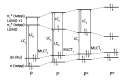
In this review, we first discuss the photophysics reported in the literature for mononuclear ruthenium complexes bearing ligands with extended aromaticity such as dipyrido[3,2-a:2',3'-c]phenazine (DPPZ), tetrapyrido[3,2-a:2',3'-c:3'',2''-h:2''',3'''-j]-phenazine (TPPHZ), tetrapyrido[3,2-a:2',3'-c:3'',2''-h:2''',3'''-j]acridine (TPAC), 1,10-phenanthrolino[5,6-b]1,4,5,8,9,12-hexaazatriphenylene (PHEHAT) 9,11,20,22-tetraaza- tetrapyrido[3,2-a:2',3'-c:3'',2''-l:2''',3'''-n]pentacene (TATPP), etc. Photophysical properties of binuclear and polynuclear complexes based on these extended ligands are then reported. We finally develop the use of binuclear complexes with extended π-systems for applications such as photocatalysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

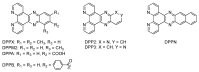


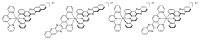

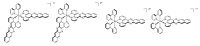








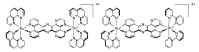

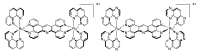
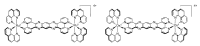






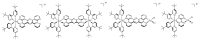




Similar articles
-
Trinuclear ruthenium dendrons based on bridging PHEHAT and TPAC ligands.Dalton Trans. 2011 Nov 28;40(44):11704-11. doi: 10.1039/c1dt10639b. Epub 2011 Aug 19. Dalton Trans. 2011. PMID: 21853187
-
Biocatalytic synthesis of the conjugated bridging ligand tetrapyrido[3,2-a:2',3'-c:3'',2''-h:2'",3'"-j]phenazine (tpphz) and a dinuclear ruthenium complex.Inorg Chem. 2003 Sep 8;42(18):5450-2. doi: 10.1021/ic034197i. Inorg Chem. 2003. PMID: 12950182
-
Complete photochromic structural changes in ruthenium(II)-diimine complexes, based on control of the excited states by metalation.Chemistry. 2013 Jul 1;19(27):8978-90. doi: 10.1002/chem.201300437. Epub 2013 May 16. Chemistry. 2013. PMID: 23681489
-
Dinuclear Ru(II) Complexes Containing Tetrapyrido[3,2-a : 2,3-c : 3,2-h : 2''',3'''-j]Phenazine Ligand for Biomedical Applications.Chembiochem. 2025 Feb 3;26(5):e202400931. doi: 10.1002/cbic.202400931. Epub 2024 Dec 20. Chembiochem. 2025. PMID: 39663208 Review.
-
Critical Overview of the Use of Ru(II) Polypyridyl Complexes as Photosensitizers in One-Photon and Two-Photon Photodynamic Therapy.Acc Chem Res. 2017 Nov 21;50(11):2727-2736. doi: 10.1021/acs.accounts.7b00180. Epub 2017 Oct 23. Acc Chem Res. 2017. PMID: 29058879 Review.
Cited by
-
Engineering Single Ni Sites on 3D Cage-like Cucurbit[n]uril Ligands for Efficient and Selective CO2 Photocatalytic Reduction.Angew Chem Int Ed Engl. 2025 Jan 27;64(5):e202417384. doi: 10.1002/anie.202417384. Epub 2024 Nov 16. Angew Chem Int Ed Engl. 2025. PMID: 39467046 Free PMC article.
-
Ultrafast excited state dynamics and light-switching of [Ru(phen)2(dppz)]2+ in G-quadruplex DNA.Commun Chem. 2021 May 14;4(1):68. doi: 10.1038/s42004-021-00507-0. Commun Chem. 2021. PMID: 36697709 Free PMC article.
-
Synthesis and Characterization of Iridium(III) Complexes with Substituted Phenylimidazo(4,5-f)1,10-phenanthroline Ancillary Ligands and Their Application in LEC Devices.Molecules. 2023 Dec 21;29(1):53. doi: 10.3390/molecules29010053. Molecules. 2023. PMID: 38202636 Free PMC article.
-
Ruthenium Complexes as Anticancer Agents: A Brief History and Perspectives.Drug Des Devel Ther. 2020 Dec 3;14:5375-5392. doi: 10.2147/DDDT.S275007. eCollection 2020. Drug Des Devel Ther. 2020. PMID: 33299303 Free PMC article. Review.
-
Editorial of Special Issue Ruthenium Complex: The Expanding Chemistry of the Ruthenium Complexes.Molecules. 2015 Sep 18;20(9):17244-74. doi: 10.3390/molecules200917244. Molecules. 2015. PMID: 26393560 Free PMC article.
References
-
- Barton J.K., Danishefsky A., Goldberg J.M. Tris(phenanthroline)ruthenium(II): Stereoselectivity in binding to DNA. J. Am. Chem. Soc. 1984;106:2172–2176. doi: 10.1021/ja00319a043. - DOI
-
- Barton J.K., Goldberg J.M., Kumar C.V., Turro N.J. Binding modes and base specificity of tris(phenanthroline)ruthenium(II) enantiomers with nucleic acids: Tuning the stereoselectivity. J. Am. Chem. Soc. 1986;108:2081–2088. doi: 10.1021/ja00268a057. - DOI
-
- Pyle A.M., Barton J.K. Probing nucleic acids with transition metal complexes. Prog. Inorg. Chem. 1990;38:413–475. doi: 10.1002/9780470166390.ch7. - DOI
-
- Moucheron C., Kirsch-De Mesmaeker A., Kelly J.M. Photophysics and Photochemistry of Metal Polypyridyl and Related Complexes with Nucleic Acids. In: Richmond J.P., editor. Less Common Metals In Proteins And Nucleic Acid Probes. Springer Berlin; Heidelberg, Germany: 1998. pp. 163–216.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

