Antioxidant and antitumor activities of new synthesized aromatic C-nucleoside derivatives
- PMID: 24759075
- PMCID: PMC6271343
- DOI: 10.3390/molecules19045163
Antioxidant and antitumor activities of new synthesized aromatic C-nucleoside derivatives
Abstract
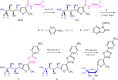
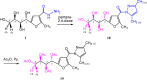
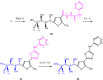
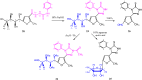
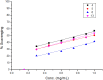
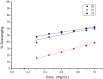
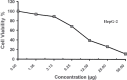
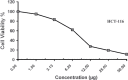
The carbohydrazide 1 was used as the precursor for the synthesis of a number of new aromatic C-nucleosides containing 1,3,4-oxadiazole 7, [1,3,4]oxadiazolo[2,3-a]isoindole 10b and pyrazole units 18. On the other hand, the thiosemicarbazone 20 was used as the key intermediate for synthesis of 1,3,4-oxadiazole and 1,2,4-triazole-3-thione derivatives 21 and 23. The antioxidant activities of the prepared compounds were evaluated. The carbohydrazide 1 in particular was found to have potent antioxidant and antitumor activity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- El-Sadek M.M., El-Dayem N.S.A., Hassan S.Y., Yacout G.A. 1,3,4-oxadiazole and selenadiazole derivatives as new C-glycosyl analogs with MAO-B, antibacterial and antifungal activities. Int. Res. J. Microbiol. 2013;4:204–219.
-
- El-Sadek M.M., Hassan S.Y., El-Dayem N.S.A., Yacout G.A. 5-(5-Aryl-1,3,4-oxadiazole-2-carbonyl)furan-3-carboxylate and new cyclic C-glycoside analogues from carbohydrate precursors with MAO-B, antimicrobial and antifungal activities. Molecules. 2012;17:7010–7027. doi: 10.3390/molecules17067010. - DOI - PMC - PubMed
-
- Nicolaou K.C., Ellery S.P., Rivas F., Saye K., Rogers E., Workinger T.J., Schallenberger M., Tawatao R., Montero A., Hessell A., et al. Synthesis and biological evaluation of 2',4'- and 3',4'-bridged nucleoside analogues. Bioorg. Med. Chem. 2011;19:5648–5669. doi: 10.1016/j.bmc.2011.07.022. - DOI - PMC - PubMed
-
- Popsavin M., Torović L., Spaić S., Stankov S., Kapor A., Tomić Z., Popsavin V. Synthesis and biological evaluation of new pyrazole- and tetrazole-related C-nucleosides with modified sugar moieties. Tetrahedron. 2002;58:569–580. doi: 10.1016/S0040-4020(01)01126-7. - DOI
-
- Kamel M.M., Ali H.I., Anwar M.M., Mohamed N.A., Soliman A.M. Synthesis, antitumor activity and molecular docking study of novel sulfonamide-schiff’s bases, thiazolidinones, benzothiazinones and their C-nucleoside derivatives. Eur. J. Med. Chem. 2010;45:572–580. doi: 10.1016/j.ejmech.2009.10.044. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

