A preliminary evaluation of efficacy and safety of Wharton's jelly mesenchymal stem cell transplantation in patients with type 2 diabetes mellitus
- PMID: 24759263
- PMCID: PMC4055092
- DOI: 10.1186/scrt446
A preliminary evaluation of efficacy and safety of Wharton's jelly mesenchymal stem cell transplantation in patients with type 2 diabetes mellitus
Abstract
Introduction: Stem cell therapy has recently been introduced to treat patients with type 2 diabetes mellitus (T2DM). However, no data are available on the efficacy and safety of allogeneic Wharton's Jelly-derived mesenchymal stem cell (WJ-MSC) transplantation in patients with T2DM. Here we performed a non-placebo controlled prospective phase I/II study to determine efficacy and safety of WJ-MSC transplantation in T2DM.
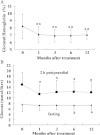
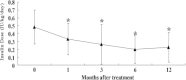
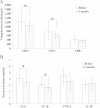
Methods: Twenty-two patients with T2DM were enrolled and received WJ-MSC transplantation through one intravenous injection and one intrapancreatic endovascular injection (catheterization). They were followed up for 12 months after transplantation. The primary endpoints were changes in the levels of glycated hemoglobin and C-peptide and the secondary endpoints included insulin dosage, fasting blood glucose (FBG), post-meal blood glucose (PBG), inflammatory markers and T lymphocyte counts.
Results: WJ-MSC transplantation significantly decreased the levels of glucose and glycated hemoglobin, improved C-peptide levels and beta cell function, and reduced markers of systemic inflammation and T lymphocyte counts. No major WJ-MSC transplantation-related adverse events occurred, but data suggest a temporary decrease in levels of C-peptide and beta cell function at one month after treatment, possibly related to intrapancreatic endovascular injection.
Conclusions: Our data demonstrate that treatment with WJ-MSCs can improve metabolic control and beta cell function in patients with T2DM. The therapeutic mechanism may involve improvements in systemic inflammation and/or immunological regulation.
Trial registration: Chinese Clinical Trial Register ChiCTR-ONC-10000985. Registered 23 September 2010.
Figures


References
-
- Kolb H, Mandrup-Poulsen T. An immune origin of type 2 diabetes? Diabetologia. 2005;48:1038–1050. - PubMed
-
- Bhansali A, Upreti V, Khandelwal N, Marwaha N, Gupta V, Sachdeva N, Sharma RR, Saluja K, Dutta P, Walia R, Minz R, Bhadada S, Das S, Ramakrishnan S. Efficacy of autologous bone marrow-derived stem cell transplantation in patients with type 2 diabetes mellitus. Stem Cells Dev. 2009;18:1407–1416. doi: 10.1089/scd.2009.0164. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

