Determination of disk diffusion and MIC quality control guidelines for GSK2140944, a novel bacterial type II topoisomerase inhibitor antimicrobial agent
- PMID: 24759716
- PMCID: PMC4097705
- DOI: 10.1128/JCM.00656-14
Determination of disk diffusion and MIC quality control guidelines for GSK2140944, a novel bacterial type II topoisomerase inhibitor antimicrobial agent
Abstract
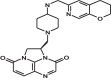
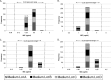
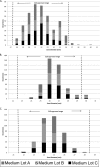
GSK2140944 is a novel bacterial type II topoisomerase inhibitor in development for the treatment of conventional and biothreat pathogens, including Gram-positive pathogens and methicillin-resistant Staphylococcus aureus. This quality control study was performed to establish ranges for selected control strains: S. aureus ATCC 29213 and ATCC 25923, Escherichia coli ATCC 25922, Haemophilus influenzae ATCC 49247, and Streptococcus pneumoniae ATCC 49619. The control ranges will be crucial for the accurate evaluation of GSK2140944 potency as it progresses through clinical trial development.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures
References
-
- Bax BD, Chan PF, Eggleston DS, Fosberry A, Gentry DR, Gorrec F, Giordano I, Hann MM, Hennessy A, Hibbs M, Huang J, Jones E, Jones J, Brown KK, Lewis CJ, May EW, Saunders MR, Singh O, Spitzfaden CE, Shen C, Shillings A, Theobald AJ, Wohlkonig A, Pearson ND, Gwynn MN. 2010. Type IIA topoisomerase inhibition by a new class of antibacterial agents. Nature 466:935–940. 10.1038/nature09197 - DOI - PubMed
-
- Bouchillon S, Hackel M, Miller LA, Scangarella-Oman NE. 2013. In vitro activity of GSK2140944, a novel topoisomerase inhibitor, against isolates associated with lower respiratory tract and skin infections, abstr F-1216 Abstr. 53rd Intersci. Conf. Antimicrob. Agents Chemother., Denver, CO, 10 to 13 September, 2013
-
- Clinical and Laboratory Standards Institute. 2008. M23–A3. Development of in vitro susceptibility testing criteria and quality control parameters; approved guideline, 3rd ed. CLSI document M23–A3. Clinical and Laboratory Standards Institute, Wayne, PA
-
- Clinical and Laboratory Standards Institute. 2012. Performance standards for antimicrobial disk susceptibility tests; approved standard—11th ed CLSI document M02-A11. Clinical and Laboratory Standards Institute, Wayne, PA
-
- Clinical and Laboratory Standards Institute. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—9th ed. CLSI document M07-A9. Clinical and Laboratory Standards Institute, Wayne, PA
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

