Non-thermal atmospheric pressure plasma preferentially induces apoptosis in p53-mutated cancer cells by activating ROS stress-response pathways
- PMID: 24759730
- PMCID: PMC3997341
- DOI: 10.1371/journal.pone.0091947
Non-thermal atmospheric pressure plasma preferentially induces apoptosis in p53-mutated cancer cells by activating ROS stress-response pathways
Abstract
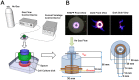
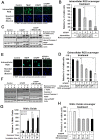
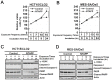
Non-thermal atmospheric pressure plasma (NTAPP) is an ionized gas at room temperature and has potential as a new apoptosis-promoting cancer therapy that acts by generating reactive oxygen species (ROS). However, it is imperative to determine its selectivity and standardize the components and composition of NTAPP. Here, we designed an NTAPP-generating apparatus combined with a He gas feeding system and demonstrated its high selectivity toward p53-mutated cancer cells. We first determined the proper conditions for NTAPP exposure to selectively induce apoptosis in cancer cells. The apoptotic effect of NTAPP was greater for p53-mutated cancer cells; artificial p53 expression in p53-negative HT29 cells decreased the pro-apoptotic effect of NTAPP. We also examined extra- and intracellular ROS levels in NTAPP-treated cells to deduce the mechanism of NTAPP action. While NTAPP-mediated increases in extracellular nitric oxide (NO) did not affect cell viability, intracellular ROS increased under NTAPP exposure and induced apoptotic cell death. This effect was dose-dependently reduced following treatment with ROS scavengers. NTAPP induced apoptosis even in doxorubicin-resistant cancer cell lines, demonstrating the feasibility of NTAPP as a potent cancer therapy. Collectively, these results strongly support the potential of NTAPP as a selective anticancer treatment, especially for p53-mutated cancer cells.
Conflict of interest statement
Figures




References
-
- Amundson SA, Myers TG, Fornace AJ Jr (1998) Roles for p53 in growth arrest and apoptosis: putting on the brakes after genotoxic stress. Oncogene 17: 3287–3299. - PubMed
-
- Fridman JS, Lowe SW (2003) Control of apoptosis by p53. Oncogene 22: 9030–9040. - PubMed
-
- Hussain SP, Amstad P, He P, Robles A, Lupold S, et al. (2004) p53-induced up-regulation of MnSOD and GPx but not catalase increases oxidative stress and apoptosis. Cancer Res 64: 2350–2356. - PubMed
-
- Yoon KA, Nakamura Y, Arakawa H (2004) Identification of ALDH4 as a p53-inducible gene and its protective role in cellular stresses. J Hum Genet 49: 134–140. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

