Crosstalk between adrenergic and toll-like receptors in human mesenchymal stem cells and keratinocytes: a recipe for impaired wound healing
- PMID: 24760207
- PMCID: PMC4039457
- DOI: 10.5966/sctm.2013-0200
Crosstalk between adrenergic and toll-like receptors in human mesenchymal stem cells and keratinocytes: a recipe for impaired wound healing
Abstract
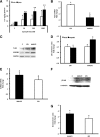
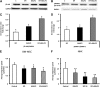
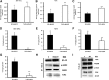
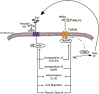
Previous studies demonstrate that skin wounds generate epinephrine (EPI) that can activate local adrenergic receptors (ARs), impairing healing. Bacterially derived activators of Toll-like receptors (TLRs) within the wound initiate inflammatory responses and can also impair healing. In this study, we examined the hypothesis that these two pathways crosstalk to one another, using EPI and macrophage-activating lipopeptide-2 (MALP2) to activate ARs and TLR2, respectively, in human bone marrow-derived mesenchymal stem cells (BM-MSCs) and neonatal keratinocytes (NHKs). BM-MSCs exposed to EPI significantly (p < .05) increased TLR2 message (sevenfold BM-MSCs), TLR2 protein (twofold), and myeloid differentiation factor 88 (MyD88) (fourfold). Conversely, activation of TLR2 by MALP2 in these cells increased β2-AR message (twofold in BM-MSCs, 2.7-fold in NHKs), β2-AR protein (2.5-fold), phosphorylation of β-AR-activated kinase (p-BARK, twofold), and induced release of EPI from both cell types (twofold). Treating cells with EPI and MALP2 together, as would be encountered in a wound, increased β2-AR and p-BARK protein expression (sixfold), impaired cell migration (BM-MSCs- 21%↓ and NHKs- 60%↓, p < .002), and resulted in a 10-fold (BM-MSCs) and 51-fold (NHKs) increase in release of IL-6 (p < .001) responses that were remarkably reduced by pretreatment with β2-AR antagonists. In vivo, EPI-stressed animals exhibited impaired healing, with elevated levels of TLR2, MyD88, and IL-6 in the wounds (p < .05) relative to nonstressed controls. Thus, our data describe a recipe for decreasing cell migration and exacerbating inflammation via novel crosstalk between the adrenergic and Toll-like receptor pathways in BM-MSCs and NHKs.
Keywords: Cell migration; Cell signaling; Mesenchymal stem cells; Stem cell-microenvironment interactions; Tissue regeneration.
©AlphaMed Press.
Figures



Similar articles
-
Bone Marrow-Derived Mesenchymal Stem Cells Promoted Cutaneous Wound Healing by Regulating Keratinocyte Migration via β2-Adrenergic Receptor Signaling.Mol Pharm. 2018 Jul 2;15(7):2513-2527. doi: 10.1021/acs.molpharmaceut.7b01138. Epub 2018 May 24. Mol Pharm. 2018. PMID: 29757659
-
Stress-mediated increases in systemic and local epinephrine impair skin wound healing: potential new indication for beta blockers.PLoS Med. 2009 Jan 13;6(1):e12. doi: 10.1371/journal.pmed.1000012. PLoS Med. 2009. PMID: 19143471 Free PMC article.
-
Beta2-adrenergic receptor activation delays wound healing.FASEB J. 2006 Jan;20(1):76-86. doi: 10.1096/fj.05-4188com. FASEB J. 2006. PMID: 16394270
-
The effects of graphene and mesenchymal stem cells in cutaneous wound healing and their putative action mechanism.Int J Nanomedicine. 2019 Apr 1;14:2281-2299. doi: 10.2147/IJN.S190928. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31015759 Free PMC article. Review.
-
Experimental models and methods for cutaneous wound healing assessment.Int J Exp Pathol. 2020 Feb;101(1-2):21-37. doi: 10.1111/iep.12346. Epub 2020 Mar 30. Int J Exp Pathol. 2020. PMID: 32227524 Free PMC article. Review.
Cited by
-
Combination product of dermal matrix, human mesenchymal stem cells, and timolol promotes diabetic wound healing in mice.Stem Cells Transl Med. 2020 Nov;9(11):1353-1364. doi: 10.1002/sctm.19-0380. Epub 2020 Jul 28. Stem Cells Transl Med. 2020. PMID: 32720751 Free PMC article.
-
Topical Anti-ulcerogenic Effect of the Beta-adrenergic Blockers on Diabetic Foot Ulcers: Recent Advances and Future Prospectives.Curr Diabetes Rev. 2024;20(8):23-37. doi: 10.2174/0115733998249061231009093006. Curr Diabetes Rev. 2024. PMID: 37867269 Review.
-
Beta-adrenergic antagonist for the healing of chronic diabetic foot ulcers: study protocol for a prospective, randomized, double-blinded, controlled and parallel-group study.Trials. 2020 Jun 8;21(1):496. doi: 10.1186/s13063-020-04413-z. Trials. 2020. PMID: 32513257 Free PMC article.
-
[Mechanisms of adrenergic β-antagonist for wounds and its application prospect in diabetic foot ulcers].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2020 Dec 15;34(12):1630-1634. doi: 10.7507/1002-1892.202002063. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2020. PMID: 33319548 Free PMC article. Review. Chinese.
-
Identification of Potential Biomarkers for Gut Barrier Failure in Broiler Chickens.Front Vet Sci. 2015 May 26;2:14. doi: 10.3389/fvets.2015.00014. eCollection 2015. Front Vet Sci. 2015. PMID: 26664943 Free PMC article.
References
-
- Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736–1743. - PubMed
-
- Brem H, Sheehan P, Boulton AJ. Protocol for treatment of diabetic foot ulcers. Am J Surg. 2004;187:1S–10S. - PubMed
-
- Davis SC, Ricotti C, Cazzaniga A, et al. Microscopic and physiologic evidence for biofilm-associated wound colonization in vivo. Wound Repair Regen. 2008;16:23–29. - PubMed
-
- Dasu MR, Isseroff RR. Toll-like receptors in wound healing: Location, accessibility, and timing. J Invest Dermatol. 2012;132:1955–1958. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

