Growth hormone secretagogue receptor deficiency in mice protects against obesity-induced hypertension
- PMID: 24760503
- PMCID: PMC4002229
- DOI: 10.1002/phy2.240
Growth hormone secretagogue receptor deficiency in mice protects against obesity-induced hypertension
Abstract
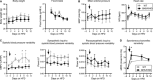
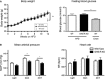
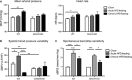
Abstract Growth hormone secretagogue receptor (GHS-R) signaling has been associated with growth hormone release, increases in food intake and pleiotropic cardiovascular effects. Recent data demonstrated that acute GHS-R antagonism leads to increases in mean arterial pressure mediated by the sympathetic nervous system in rats; a highly undesirable effect if GHS-R antagonism was to be used as a therapeutic approach to reducing food intake in an already obese, hypertensive patient population. However, our data in conscious, freely moving GHS-R deficient mice demonstrate that chronic absence of GHS-R signaling is protective against obesity-induced hypertension. GHS-R deficiency leads to reduced systolic blood pressure variability (SBPV); in response to acute high-fat diet (HFD)-feeding, increases in the sympathetic control of SBPV are suppressed in GHS-R KO mice. Our data further suggest that GHS-R signaling dampens the immediate HFD-mediated increase in spontaneous baroreflex sensitivity. In diet-induced obesity, absence of GHS-R signaling leads to reductions in obesity-mediated hypertension and tachycardia. Collectively, our findings thus suggest that chronic blockade of GHS-R signaling may not result in adverse cardiovascular effects in obesity.
Keywords: Diet‐induced obesity; ghrelin; growth hormone secretagogue receptor; hypertension.
Figures

Similar articles
-
Antagonism of ghrelin receptor reduces food intake and body weight gain in mice.Gut. 2003 Jul;52(7):947-52. doi: 10.1136/gut.52.7.947. Gut. 2003. PMID: 12801949 Free PMC article.
-
Effect of ghrelin receptor agonist and antagonist on the activity of arcuate nucleus tyrosine hydroxylase containing neurons in C57BL/6 male mice exposed to normal or high fat diet.J Physiol Pharmacol. 2014 Aug;65(4):477-86. J Physiol Pharmacol. 2014. PMID: 25179080
-
GHS-R in brown fat potentiates differential thermogenic responses under metabolic and thermal stresses.PLoS One. 2021 Apr 1;16(4):e0249420. doi: 10.1371/journal.pone.0249420. eCollection 2021. PLoS One. 2021. PMID: 33793646 Free PMC article.
-
The growth hormone secretagogue receptor (Ghs-R).Curr Pharm Des. 2012;18(31):4749-54. doi: 10.2174/138161212803216906. Curr Pharm Des. 2012. PMID: 22632856 Review.
-
Structure and regulation of the growth hormone secretagogue receptor.Minerva Endocrinol. 2002 Dec;27(4):243-56. Minerva Endocrinol. 2002. PMID: 12511847 Review.
Cited by
-
Improved glucose homeostasis in male obese Zucker rats coincides with enhanced baroreflexes and activation of the nucleus tractus solitarius.Am J Physiol Regul Integr Comp Physiol. 2018 Dec 1;315(6):R1195-R1209. doi: 10.1152/ajpregu.00195.2018. Epub 2018 Sep 26. Am J Physiol Regul Integr Comp Physiol. 2018. PMID: 30256679 Free PMC article.
References
-
- Baudrie, V. , Laude D., and Elghozi J. L.. 2007. Optimal frequency ranges for extracting information on cardiovascular autonomic control from the blood pressure and pulse interval spectrograms in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292:R904–R912. - PubMed
-
- Benso, A. , Broglio F., Marafetti L., Lucatello B., Seardo M. A., Granata R., et al. 2004. Ghrelin and synthetic growth hormone secretagogues are cardioactive molecules with identities and differences. Semin. Vasc. Med. 4:107–114. - PubMed
-
- Bisi, G. , Podio V., Valetto M. R., Broglio F., Bertuccio G., Del Rio G., et al. 1999. Acute cardiovascular and hormonal effects of GH and hexarelin, a synthetic GH‐releasing peptide, in humans. J. Endocrinol. Invest. 22:266–272. - PubMed
-
- Callaghan, B. , Hunne B., Hirayama H., Sartor D. M., Nguyen T. V., Abogadie F. C., et al. 2012. Sites of action of ghrelin receptor ligands in cardiovascular control. Am. J. Physiol. Heart Circ. Physiol. 303:H1011–H1021. - PubMed
-
- Dangardt, F. , Volkmann R., Chen Y., Osika W., Marild S., and Friberg P.. 2011. Reduced cardiac vagal activity in obese children and adolescents. Clin. Physiol. Funct. Imaging 31:108–113. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

