Continuous postnatal neurogenesis contributes to formation of the olfactory bulb neural circuits and flexible olfactory associative learning
- PMID: 24760839
- PMCID: PMC6608281
- DOI: 10.1523/JNEUROSCI.0674-14.2014
Continuous postnatal neurogenesis contributes to formation of the olfactory bulb neural circuits and flexible olfactory associative learning
Abstract
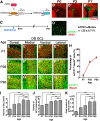
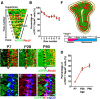
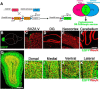
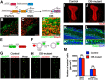
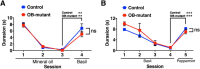
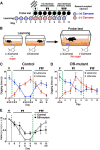
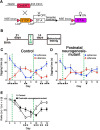
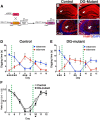
The olfactory bulb (OB) is one of the two major loci in the mammalian brain where newborn neurons are constantly integrated into the neural circuit during postnatal life. Newborn neurons are generated from neural stem cells in the subventricular zone (SVZ) of the lateral ventricle and migrate to the OB through the rostral migratory stream. The majority of these newborn neurons differentiate into inhibitory interneurons, such as granule cells and periglomerular cells. It has been reported that prolonged supply of newborn neurons leads to continuous addition/turnover of the interneuronal populations and contributes to functional integrity of the OB circuit. However, it is not still clear how and to what extent postnatal-born neurons contribute to OB neural circuit formation, and the functional role of postnatal neurogenesis in odor-related behaviors remains elusive. To address this question, here by using genetic strategies, we first determined the unique integration mode of newly born interneurons during postnatal development of the mouse OB. We then manipulated these interneuron populations and found that continuous postnatal neurogenesis in the SVZ-OB plays pivotal roles in flexible olfactory associative learning and memory.
Keywords: dentate gyrus; hippocampus; learning; neural stem cells; neurogenesis; olfactory bulb.
Figures

Similar articles
-
Intrinsic Neuronal Activity during Migration Controls the Recruitment of Specific Interneuron Subtypes in the Postnatal Mouse Olfactory Bulb.J Neurosci. 2021 Mar 24;41(12):2630-2644. doi: 10.1523/JNEUROSCI.1960-20.2021. Epub 2021 Feb 3. J Neurosci. 2021. PMID: 33536198 Free PMC article.
-
Interneurons produced in adulthood are required for the normal functioning of the olfactory bulb network and for the execution of selected olfactory behaviors.J Neurosci. 2009 Dec 2;29(48):15245-57. doi: 10.1523/JNEUROSCI.3606-09.2009. J Neurosci. 2009. PMID: 19955377 Free PMC article.
-
The Role of Adult-Born Neurons in the Constantly Changing Olfactory Bulb Network.Neural Plast. 2016;2016:1614329. doi: 10.1155/2016/1614329. Epub 2015 Dec 29. Neural Plast. 2016. PMID: 26839709 Free PMC article. Review.
-
Postnatal neurogenesis and gliogenesis in the olfactory bulb from NG2-expressing progenitors of the subventricular zone.J Neurosci. 2004 Nov 17;24(46):10530-41. doi: 10.1523/JNEUROSCI.3572-04.2004. J Neurosci. 2004. PMID: 15548668 Free PMC article.
-
The functional significance of newly born neurons integrated into olfactory bulb circuits.Front Neurosci. 2014 May 26;8:121. doi: 10.3389/fnins.2014.00121. eCollection 2014. Front Neurosci. 2014. PMID: 24904263 Free PMC article. Review.
Cited by
-
Pbx1 is required for adult subventricular zone neurogenesis.Development. 2016 Jul 1;143(13):2281-91. doi: 10.1242/dev.128033. Epub 2016 May 25. Development. 2016. PMID: 27226325 Free PMC article.
-
In vitro Time-lapse Imaging of Primary Cilium in Migrating Neuroblasts.Bio Protoc. 2020 Nov 20;10(22):e3823. doi: 10.21769/BioProtoc.3823. eCollection 2020 Nov 20. Bio Protoc. 2020. PMID: 33659475 Free PMC article.
-
Neurogranin Regulates Adult-Born Olfactory Granule Cell Spine Density and Odor-Reward Associative Memory in Mice.Int J Mol Sci. 2021 Apr 20;22(8):4269. doi: 10.3390/ijms22084269. Int J Mol Sci. 2021. PMID: 33924098 Free PMC article.
-
Control of adult neurogenesis by programmed cell death in the mammalian brain.Mol Brain. 2016 Apr 21;9:43. doi: 10.1186/s13041-016-0224-4. Mol Brain. 2016. PMID: 27098178 Free PMC article. Review.
-
Retrieval of olfactory fear memory alters cell proliferation and expression of pCREB and pMAPK in the corticomedial amygdala and piriform cortex.Chem Senses. 2022 Jan 1;47:bjac021. doi: 10.1093/chemse/bjac021. Chem Senses. 2022. PMID: 35997758 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
