Activation of dopaminergic D2/D3 receptors modulates dorsoventral connectivity in the hippocampus and reverses the impairment of working memory after nerve injury
- PMID: 24760846
- PMCID: PMC6608290
- DOI: 10.1523/JNEUROSCI.0021-14.2014
Activation of dopaminergic D2/D3 receptors modulates dorsoventral connectivity in the hippocampus and reverses the impairment of working memory after nerve injury
Abstract
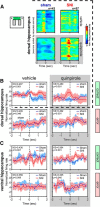
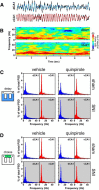
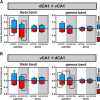
Dopamine plays an important role in several forms of synaptic plasticity in the hippocampus, a crucial brain structure for working memory (WM) functioning. In this study, we evaluated whether the working-memory impairment characteristic of animal models of chronic pain is dependent on hippocampal dopaminergic signaling. To address this issue, we implanted multichannel arrays of electrodes in the dorsal and ventral hippocampal CA1 region of rats and recorded the neuronal activity during a food-reinforced spatial WM task of trajectory alternation. Within-subject behavioral performance and patterns of dorsoventral neuronal activity were assessed before and after the onset of persistent neuropathic pain using the Spared Nerve Injury (SNI) model of neuropathic pain. Our results show that the peripheral nerve lesion caused a disruption in WM and in hippocampus spike activity and that this disruption was reversed by the systemic administration of the dopamine D2/D3 receptor agonist quinpirole (0.05 mg/kg). In SNI animals, the administration of quinpirole restored both the performance-related and the task-related spike activity to the normal range characteristic of naive animals, whereas quinpirole in sham animals caused the opposite effect. Quinpirole also reversed the abnormally low levels of hippocampus dorsoventral connectivity and phase coherence. Together with our finding of changes in gene expression of dopamine receptors and modulators after the onset of the nerve injury model, these results suggest that disruption of the dopaminergic balance in the hippocampus may be crucial for the clinical neurological and cognitive deficits observed in patients with painful syndromes.
Keywords: awake animal physiology; dopamine; hippocampus; neuropathy; pain; working memory.
Figures






Similar articles
-
Blockade of dopamine D2 receptors disrupts intrahippocampal connectivity and enhances pain-related working memory deficits in neuropathic pain rats.Eur J Pain. 2018 May;22(5):1002-1015. doi: 10.1002/ejp.1186. Epub 2018 Jan 26. Eur J Pain. 2018. PMID: 29377353
-
Impaired spatial memory performance in a rat model of neuropathic pain is associated with reduced hippocampus-prefrontal cortex connectivity.J Neurosci. 2013 Feb 6;33(6):2465-80. doi: 10.1523/JNEUROSCI.5197-12.2013. J Neurosci. 2013. PMID: 23392675 Free PMC article.
-
Ventral hippocampal dopamine D1 and D2 systems and spatial working memory in rats.Neuroscience. 1999 Mar;89(3):743-9. doi: 10.1016/s0306-4522(98)00346-7. Neuroscience. 1999. PMID: 10199609
-
Spatial working memory deficits in GluA1 AMPA receptor subunit knockout mice reflect impaired short-term habituation: evidence for Wagner's dual-process memory model.Neuropsychologia. 2010 Jul;48(8):2303-15. doi: 10.1016/j.neuropsychologia.2010.03.018. Epub 2010 Mar 27. Neuropsychologia. 2010. PMID: 20350557 Free PMC article. Review.
-
Clinical significance of knowledge about the structure, function, and impairments of working memory.Med Sci Monit. 2013 May 3;19:327-38. doi: 10.12659/MSM.883900. Med Sci Monit. 2013. PMID: 23645218 Free PMC article. Review.
Cited by
-
Anterior-Posterior Hippocampal Dynamics Support Working Memory Processing.J Neurosci. 2022 Jan 19;42(3):443-453. doi: 10.1523/JNEUROSCI.1287-21.2021. Epub 2021 Nov 24. J Neurosci. 2022. PMID: 34819340 Free PMC article.
-
Altered Brain Expression of DNA Methylation and Hydroxymethylation Epigenetic Enzymes in a Rat Model of Neuropathic Pain.Int J Mol Sci. 2023 Apr 15;24(8):7305. doi: 10.3390/ijms24087305. Int J Mol Sci. 2023. PMID: 37108466 Free PMC article.
-
Effect of Motor Impairment on Analgesic Efficacy of Dopamine D2/3 Receptors in a Rat Model of Neuropathy.J Exp Neurosci. 2016 Apr 6;10:51-7. doi: 10.4137/JEN.S36492. eCollection 2016. J Exp Neurosci. 2016. PMID: 27081316 Free PMC article.
-
Overlapping signatures of chronic pain in the DNA methylation landscape of prefrontal cortex and peripheral T cells.Sci Rep. 2016 Jan 28;6:19615. doi: 10.1038/srep19615. Sci Rep. 2016. PMID: 26817950 Free PMC article.
-
Cortico-limbic pain mechanisms.Neurosci Lett. 2019 May 29;702:15-23. doi: 10.1016/j.neulet.2018.11.037. Epub 2018 Nov 29. Neurosci Lett. 2019. PMID: 30503916 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
