Source-reconstruction of event-related fields reveals hyperfunction and hypofunction of cortical circuits in antipsychotic-naive, first-episode schizophrenia patients during Mooney face processing
- PMID: 24760850
- PMCID: PMC6608292
- DOI: 10.1523/JNEUROSCI.3752-13.2014
Source-reconstruction of event-related fields reveals hyperfunction and hypofunction of cortical circuits in antipsychotic-naive, first-episode schizophrenia patients during Mooney face processing
Abstract
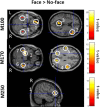
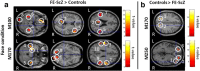
Schizophrenia is characterized by dysfunctions in neural circuits that can be investigated with electrophysiological methods, such as EEG and MEG. In the present human study, we examined event-related fields (ERFs), in a sample of medication-naive, first-episode schizophrenia (FE-ScZ) patients (n = 14) and healthy control participants (n = 17) during perception of Mooney faces to investigate the integrity of neuromagnetic responses and their experience-dependent modification. ERF responses were analyzed for M100, M170, and M250 components at the sensor and source levels. In addition, we analyzed peak latency and adaptation effects due to stimulus repetition. FE-ScZ patients were characterized by significantly impaired sensory processing, as indicated by a reduced discrimination index (A'). At the sensor level, M100 and M170 responses in FE-ScZ were within the normal range, whereas the M250 response was impaired. However, source localization revealed widespread elevated activity for M100 and M170 in FE-ScZ and delayed peak latencies for the M100 and M250 responses. In addition, M170 source activity in FE-ScZ was not modulated by stimulus repetitions. The present findings suggest that neural circuits in FE-ScZ may be characterized by a disturbed balance between excitation and inhibition that could lead to a failure to gate information flow and abnormal spreading of activity, which is compatible with dysfunctional glutamatergic neurotransmission.
Keywords: ERFs; M170; MEG; Mooney faces; face processing; first-episode psychosis.
Figures






Similar articles
-
Evidence for dysregulated high-frequency oscillations during sensory processing in medication-naïve, first episode schizophrenia.Schizophr Res. 2013 Nov;150(2-3):519-25. doi: 10.1016/j.schres.2013.08.023. Epub 2013 Sep 7. Schizophr Res. 2013. PMID: 24016727
-
Brain responses to repetitions of human and animal faces, inverted faces, and objects: an MEG study.Brain Res. 2007 Dec 12;1184:226-33. doi: 10.1016/j.brainres.2007.09.079. Epub 2007 Oct 9. Brain Res. 2007. PMID: 17976538
-
Inversion and contrast-reversal effects on face processing assessed by MEG.Brain Res. 2006 Oct 18;1115(1):108-20. doi: 10.1016/j.brainres.2006.07.072. Epub 2006 Aug 22. Brain Res. 2006. PMID: 16930564
-
A magnetoencephalographic study of face processing: M170, gamma-band oscillations and source localization.Hum Brain Mapp. 2013 Aug;34(8):1783-95. doi: 10.1002/hbm.22028. Epub 2012 Mar 16. Hum Brain Mapp. 2013. PMID: 22422432 Free PMC article.
-
Towards a neurodynamical understanding of the prodrome in schizophrenia.Neuroimage. 2019 Apr 15;190:144-153. doi: 10.1016/j.neuroimage.2017.11.026. Epub 2017 Nov 22. Neuroimage. 2019. PMID: 29175199 Review.
Cited by
-
Face pareidolia is enhanced by 40 Hz transcranial alternating current stimulation (tACS) of the face perception network.Sci Rep. 2023 Feb 4;13(1):2035. doi: 10.1038/s41598-023-29124-8. Sci Rep. 2023. PMID: 36739325 Free PMC article. Clinical Trial.
-
Neurophysiological Face Processing Deficits in Patients With Chronic Schizophrenia: An MEG Study.Front Psychiatry. 2020 Sep 3;11:554844. doi: 10.3389/fpsyt.2020.554844. eCollection 2020. Front Psychiatry. 2020. PMID: 33101080 Free PMC article.
-
Preliminary Evidence of "Other-Race Effect"-Like Behavior Induced by Cathodal-tDCS over the Right Occipital Cortex, in the Absence of Overall Effects on Face/Object Processing.Front Neurosci. 2017 Nov 30;11:661. doi: 10.3389/fnins.2017.00661. eCollection 2017. Front Neurosci. 2017. PMID: 29249931 Free PMC article.
-
Familiarity is not notoriety: phenomenological accounts of face recognition.Front Hum Neurosci. 2014 Sep 1;8:672. doi: 10.3389/fnhum.2014.00672. eCollection 2014. Front Hum Neurosci. 2014. PMID: 25225476 Free PMC article.
-
Computerized Assessment of Psychosis Risk.J Psychiatr Brain Sci. 2021;6(3):e210011. doi: 10.20900/jpbs.20210011. Epub 2021 Jun 29. J Psychiatr Brain Sci. 2021. PMID: 34307899 Free PMC article.
References
-
- Anderson MJ, Ter Braak CJF. Permutation tests for multi-factorial analysis of variance. Journal of Statistical Computation Simulation. 2003;78:85–113.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
