Modulation dynamics in the orofacial sensorimotor cortex during motor skill acquisition
- PMID: 24760857
- PMCID: PMC4401283
- DOI: 10.1523/JNEUROSCI.4367-13.2014
Modulation dynamics in the orofacial sensorimotor cortex during motor skill acquisition
Abstract
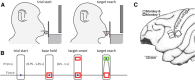
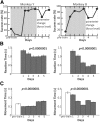
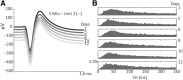
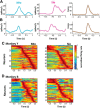
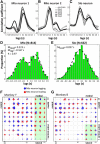
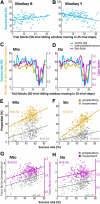
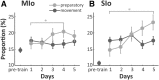
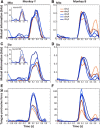
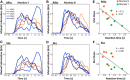
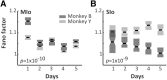
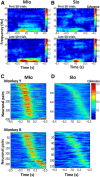
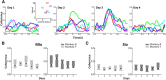
The orofacial sensorimotor cortex is known to play a role in motor learning. However, how motor learning changes the dynamics of neuronal activity and whether these changes differ between orofacial primary motor (MIo) and somatosensory (SIo) cortices remain unknown. To address these questions, we used chronically implanted microelectrode arrays to track learning-induced changes in the activity of simultaneously recorded neurons in MIo and SIo as two naive monkeys (Macaca mulatta) were trained in a novel tongue-protrusion task. Over a period of 8-12 d, the monkeys showed behavioral improvements in task performance that were accompanied by rapid and long-lasting changes in neuronal responses in MIo and SIo occurring in parallel: (1) increases in the proportion of task-modulated neurons, (2) increases in the mutual information between tongue-protrusive force and spiking activity, (3) reductions in the across-trial firing rate variability, and (4) transient increases in coherent firing of neuronal pairs. More importantly, the time-resolved mutual information in MIo and SIo exhibited temporal alignment. While showing parallel changes, MIo neurons exhibited a bimodal distribution of peak correlation lag times between spiking activity and force, whereas SIo neurons showed a unimodal distribution. Moreover, coherent activity between pairs of MIo neurons was higher and centered around force onset compared with pairwise coherence of SIo neurons. Overall, the results suggest that the neuroplasticity in MIo and SIo occurring in parallel serves as a substrate for linking sensation and movement during sensorimotor learning, whereas the differing dynamic organizations reflect specific ways to control movement parameters as learning progresses.
Keywords: electrophysiology; information; learning; motor cortex; orofacial; somatosensory cortex.
Figures
Similar articles
-
Primary sensorimotor cortex exhibits complex dependencies of spike-field coherence on neuronal firing rates, field power, and behavior.J Neurophysiol. 2018 Jul 1;120(1):226-238. doi: 10.1152/jn.00037.2018. Epub 2018 Mar 28. J Neurophysiol. 2018. PMID: 29589815 Free PMC article.
-
Directional information from neuronal ensembles in the primate orofacial sensorimotor cortex.J Neurophysiol. 2013 Sep;110(6):1357-69. doi: 10.1152/jn.00144.2013. Epub 2013 Jun 19. J Neurophysiol. 2013. PMID: 23785133
-
Primary motor and sensory cortical areas communicate via spatiotemporally coordinated networks at multiple frequencies.Proc Natl Acad Sci U S A. 2016 May 3;113(18):5083-8. doi: 10.1073/pnas.1600788113. Epub 2016 Apr 18. Proc Natl Acad Sci U S A. 2016. PMID: 27091982 Free PMC article.
-
Chapter 5--face sensorimotor cortex: its role and neuroplasticity in the control of orofacial movements.Prog Brain Res. 2011;188:71-82. doi: 10.1016/B978-0-444-53825-3.00010-3. Prog Brain Res. 2011. PMID: 21333803 Review.
-
Properties and plasticity of the primate somatosensory and motor cortex related to orofacial sensorimotor function.Clin Exp Pharmacol Physiol. 2005 Jan-Feb;32(1-2):109-14. doi: 10.1111/j.1440-1681.2005.04137.x. Clin Exp Pharmacol Physiol. 2005. PMID: 15730444 Review.
Cited by
-
Interhemispheric connectivity of primary sensory cortex is associated with motor impairment after stroke.Sci Rep. 2018 Aug 22;8(1):12601. doi: 10.1038/s41598-018-29751-6. Sci Rep. 2018. PMID: 30135496 Free PMC article.
-
Primary sensorimotor cortex exhibits complex dependencies of spike-field coherence on neuronal firing rates, field power, and behavior.J Neurophysiol. 2018 Jul 1;120(1):226-238. doi: 10.1152/jn.00037.2018. Epub 2018 Mar 28. J Neurophysiol. 2018. PMID: 29589815 Free PMC article.
-
Neural Efficiency and Acquired Motor Skills: An fMRI Study of Expert Athletes.Front Psychol. 2019 Dec 6;10:2752. doi: 10.3389/fpsyg.2019.02752. eCollection 2019. Front Psychol. 2019. PMID: 31866917 Free PMC article.
-
Movements following force-field adaptation are aligned with altered sense of limb position.Exp Brain Res. 2019 May;237(5):1303-1313. doi: 10.1007/s00221-019-05509-y. Epub 2019 Mar 12. Exp Brain Res. 2019. PMID: 30863880 Free PMC article.
-
Combination of jaw and tongue movement training influences neuroplasticity of corticomotor pathways in humans.Exp Brain Res. 2019 Oct;237(10):2559-2571. doi: 10.1007/s00221-019-05610-2. Epub 2019 Jul 25. Exp Brain Res. 2019. PMID: 31346648
References
-
- Abbott LF, Song S. Temporally asymmetric Hebbian learning, spike timing and neuronal response variability. In: Kearns MS, Solla SA, Cohn DA, editors. Advances in neural information processing systems. Ed 11. Cambridge, MA: Massachusetts Institute of Technology; 1999.
-
- Aertsen A, Vaadia E, Abeles M, Ahissar E, Bergman H, Karmon B, Lavner Y, Margalit E, Nelken I, Rotter S. Neural interactions in the frontal cortex of a behaving monkey: signs of dependence on stimulus context and behavioral state. J Hirnforsch. 1991;32:735–743. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
