The generation of antiphase oscillations and synchrony by a rebound-based vertebrate central pattern generator
- PMID: 24760866
- PMCID: PMC3996224
- DOI: 10.1523/JNEUROSCI.4198-13.2014
The generation of antiphase oscillations and synchrony by a rebound-based vertebrate central pattern generator
Abstract
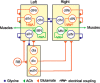
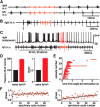
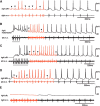
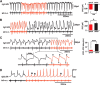
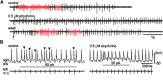
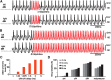
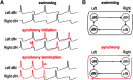
Many neural circuits are capable of generating multiple stereotyped outputs after different sensory inputs or neuromodulation. We have previously identified the central pattern generator (CPG) for Xenopus tadpole swimming that involves antiphase oscillations of activity between the left and right sides. Here we analyze the cellular basis for spontaneous left-right motor synchrony characterized by simultaneous bursting on both sides at twice the swimming frequency. Spontaneous synchrony bouts are rare in most tadpoles, and they instantly emerge from and switch back to swimming, most frequently within the first second after skin stimulation. Analyses show that only neurons that are active during swimming fire action potentials in synchrony, suggesting both output patterns derive from the same neural circuit. The firing of excitatory descending interneurons (dINs) leads that of other types of neurons in synchrony as it does in swimming. During synchrony, the time window between phasic excitation and inhibition is 7.9 ± 1 ms, shorter than that in swimming (41 ± 2.3 ms). The occasional, extra midcycle firing of dINs during swimming may initiate synchrony, and mismatches of timing in the left and right activity can switch synchrony back to swimming. Computer modeling supports these findings by showing that the same neural network, in which reciprocal inhibition mediates rebound firing, can generate both swimming and synchrony without circuit reconfiguration. Modeling also shows that lengthening the time window between phasic excitation and inhibition by increasing dIN synaptic/conduction delay can improve the stability of synchrony.
Keywords: central pattern generator; locomotion; oscillations; spinal cord; swimming; synchrony.
Figures



References
-
- Beato M, Nistri A. Interaction between disinhibited bursting and fictive locomotor patterns in the rat isolated spinal cord. J Neurophysiol. 1999;82:2029–2038. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
