Mutations in HIV-1 reverse transcriptase affect the errors made in a single cycle of viral replication
- PMID: 24760888
- PMCID: PMC4054409
- DOI: 10.1128/JVI.00302-14
Mutations in HIV-1 reverse transcriptase affect the errors made in a single cycle of viral replication
Abstract
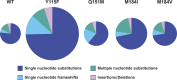
The genetic variation in HIV-1 in patients is due to the high rate of viral replication, the high viral load, and the errors made during viral replication. Some of the mutations in reverse transcriptase (RT) that alter the deoxynucleoside triphosphate (dNTP)-binding pocket, including those that confer resistance to nucleoside/nucleotide analogs, affect dNTP selection during replication. The effects of mutations in RT on the spectrum (nature, position, and frequency) of errors made in vivo are poorly understood. We previously determined the mutation rate and the frequency of different types of mutations and identified hot spots for mutations in a lacZα (the α complementing region of lacZ) reporter gene carried by an HIV-1 vector that replicates using wild-type RT. We show here that four mutations (Y115F, M184V, M184I, and Q151M) in the dNTP-binding pocket of RT that had relatively small effects on the overall HIV-1 mutation rate (less than 3-fold compared to the wild type) significantly increased mutations at some specific positions in the lacZα reporter gene. We also show that changes in a sequence that flanks the reporter gene can affect the mutations that arise in the reporter. These data show that changes either in HIV-1 RT or in the sequence of the nucleic acid template can affect the spectrum of mutations made during viral replication. This could, by implication, affect the generation of drug-resistant mutants and immunological-escape mutants in patients.
Importance: RT is the viral enzyme that converts the RNA genome of HIV into DNA. Errors made during replication allow the virus to escape from the host's immune system and to develop resistance to the available anti-HIV drugs. We show that four different mutations in RT which are known to be associated with resistance to anti-RT drugs modestly increased the overall frequency of errors made during viral replication. However, the increased errors were not uniformly distributed; the additional errors occurred at a small number of positions (hot spots). Moreover, some of the RT mutations preferentially affected the nature of the errors that were made (some RT mutations caused an increase in insertion and deletion errors; others caused an increase in substitution errors). We also show that sequence changes in a region adjacent to a target gene can affect the errors made within the target gene.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

