Host and viral determinants of Mx2 antiretroviral activity
- PMID: 24760893
- PMCID: PMC4097781
- DOI: 10.1128/JVI.00214-14
Host and viral determinants of Mx2 antiretroviral activity
Abstract
Myxovirus resistance 2 (Mx2/MxB) has recently been uncovered as an effector of the anti-HIV-1 activity of type I interferons (IFNs) that inhibits HIV-1 at an early stage postinfection, after reverse transcription but prior to proviral integration into host DNA. The mechanistic details of Mx2 antiviral activity are not yet understood, but a few substitutions in the HIV-1 capsid have been shown to confer resistance to Mx2. Through a combination of in vitro evolution and unbiased mutagenesis, we further map the determinants of sensitivity to Mx2 and reveal that multiple capsid (CA) surfaces define sensitivity to Mx2. Intriguingly, we reveal an unanticipated sensitivity determinant within the C-terminal domain of capsid. We also report that Mx2s derived from multiple primate species share the capacity to potently inhibit HIV-1, whereas selected nonprimate orthologs have no such activity. Like TRIM5α, another CA targeting antiretroviral protein, primate Mx2s exhibit species-dependent variation in antiviral specificity against at least one extant virus and multiple HIV-1 capsid mutants. Using a combination of chimeric Mx2 proteins and evolution-guided approaches, we reveal that a single residue close to the N terminus that has evolved under positive selection can determine antiviral specificity. Thus, the variable N-terminal region can define the spectrum of viruses inhibited by Mx2. Importance: Type I interferons (IFNs) inhibit the replication of most mammalian viruses. IFN stimulation upregulates hundreds of different IFN-stimulated genes (ISGs), but it is often unclear which ISGs are responsible for inhibition of a given virus. Recently, Mx2 was identified as an ISG that contributes to the inhibition of HIV-1 replication by type I IFN. Thus, Mx2 might inhibit HIV-1 replication in patients, and this inhibitory action might have therapeutic potential. The mechanistic details of how Mx2 inhibits HIV-1 are currently unclear, but the HIV-1 capsid protein is the likely viral target. Here, we determine the regions of capsid that specify sensitivity to Mx2. We demonstrate that Mx2 from multiple primates can inhibit HIV-1, whereas Mx2 from other mammals (dogs and sheep) cannot. We also show that primate variants of Mx2 differ in the spectrum of lentiviruses they inhibit and that a single residue in Mx2 can determine this antiviral specificity.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures


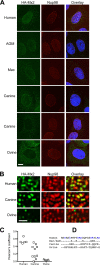


References
-
- Ho DD, Hartshorn KL, Rota TR, Andrews CA, Kaplan JC, Schooley RT, Hirsch MS. 1985. Recombinant human interferon alfa-A suppresses HTLV-III replication in vitro. Lancet i:602–604 - PubMed
-
- Asmuth DM, Murphy RL, Rosenkranz SL, Lertora JJ, Kottilil S, Cramer Y, Chan ES, Schooley RT, Rinaldo CR, Thielman N, Li XD, Wahl SM, Shore J, Janik J, Lempicki RA, Simpson Y, Pollard RB. 2010. Safety, tolerability, and mechanisms of antiretroviral activity of pegylated interferon alfa-2a in HIV-1-monoinfected participants: a phase II clinical trial. J. Infect. Dis. 201:1686–1696. 10.1086/652420 - DOI - PMC - PubMed
-
- Kirmaier A, Wu F, Newman RM, Hall LR, Morgan JS, O'Connor S, Marx PA, Meythaler M, Goldstein S, Buckler-White A, Kaur A, Hirsch VM, Johnson WE. 2010. TRIM5 suppresses cross-species transmission of a primate immunodeficiency virus and selects for emergence of resistant variants in the new species. PLoS Biol. 8:1–12. 10.1371/journal.pbio.1000462 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

