Ghrelin mimics fasting to enhance human hedonic, orbitofrontal cortex, and hippocampal responses to food
- PMID: 24760977
- PMCID: PMC6410902
- DOI: 10.3945/ajcn.113.075291
Ghrelin mimics fasting to enhance human hedonic, orbitofrontal cortex, and hippocampal responses to food
Abstract
Background: Ghrelin, which is a stomach-derived hormone, increases with fasting and energy restriction and may influence eating behaviors through brain hedonic reward-cognitive systems. Therefore, changes in plasma ghrelin might mediate counter-regulatory responses to a negative energy balance through changes in food hedonics.
Objective: We investigated whether ghrelin administration (exogenous hyperghrelinemia) mimics effects of fasting (endogenous hyperghrelinemia) on the hedonic response and activation of brain-reward systems to food.
Design: In a crossover design, 22 healthy, nonobese adults (17 men) underwent a functional magnetic resonance imaging (fMRI) food-picture evaluation task after a 16-h overnight fast (Fasted-Saline) or after eating breakfast 95 min before scanning (730 kcal, 14% protein, 31% fat, and 55% carbohydrate) and receiving a saline (Fed-Saline) or acyl ghrelin (Fed-Ghrelin) subcutaneous injection before scanning. One male subject was excluded from the fMRI analysis because of excess head motion, which left 21 subjects with brain-activation data.
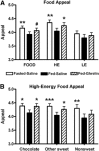
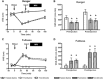
Results: Compared with the Fed-Saline visit, both ghrelin administration to fed subjects (Fed-Ghrelin) and fasting (Fasted-Saline) significantly increased the appeal of high-energy foods and associated orbitofrontal cortex activation. Both fasting and ghrelin administration also increased hippocampus activation to high-energy- and low-energy-food pictures. These similar effects of endogenous and exogenous hyperghrelinemia were not explicable by consistent changes in glucose, insulin, peptide YY, and glucagon-like peptide-1. Neither ghrelin administration nor fasting had any significant effect on nucleus accumbens, caudate, anterior insula, or amygdala activation during the food-evaluation task or on auditory, motor, or visual cortex activation during a control task.
Conclusions: Ghrelin administration and fasting have similar acute stimulatory effects on hedonic responses and the activation of corticolimbic reward-cognitive systems during food evaluations. Similar effects of recurrent or chronic hyperghrelinemia on an anticipatory food reward may contribute to the negative impact of skipping breakfast on dietary habits and body weight and the long-term failure of energy restriction for weight loss.
© 2014 American Society for Nutrition.
Figures



Similar articles
-
The corticosteroid prednisolone increases amygdala and insula reactivity to food approach signals in healthy young men.Psychoneuroendocrinology. 2019 Jan;99:154-165. doi: 10.1016/j.psyneuen.2018.09.007. Epub 2018 Sep 8. Psychoneuroendocrinology. 2019. PMID: 30245328 Clinical Trial.
-
Abnormal relationships between the neural response to high- and low-calorie foods and endogenous acylated ghrelin in women with active and weight-recovered anorexia nervosa.Psychiatry Res. 2014 Aug 30;223(2):94-103. doi: 10.1016/j.pscychresns.2014.04.015. Epub 2014 May 6. Psychiatry Res. 2014. PMID: 24862390 Free PMC article.
-
Fasting biases brain reward systems towards high-calorie foods.Eur J Neurosci. 2009 Oct;30(8):1625-35. doi: 10.1111/j.1460-9568.2009.06949.x. Epub 2009 Oct 7. Eur J Neurosci. 2009. PMID: 19811532
-
Ghrelin, appetite and critical illness.Curr Opin Crit Care. 2012 Apr;18(2):199-205. doi: 10.1097/MCC.0b013e3283514b01. Curr Opin Crit Care. 2012. PMID: 22322262 Review.
-
Gaining new insights into food reward with functional neuroimaging.Forum Nutr. 2010;63:152-163. doi: 10.1159/000264403. Epub 2009 Nov 27. Forum Nutr. 2010. PMID: 19955783 Review.
Cited by
-
Preliminary evidence of acylated ghrelin association with depression severity in postmenopausal women.Sci Rep. 2021 Mar 5;11(1):5319. doi: 10.1038/s41598-021-84431-2. Sci Rep. 2021. PMID: 33674672 Free PMC article.
-
Neural activation of regions involved in food reward and cognitive control in young females with anorexia nervosa and atypical anorexia nervosa versus healthy controls.Transl Psychiatry. 2023 Jun 23;13(1):220. doi: 10.1038/s41398-023-02494-3. Transl Psychiatry. 2023. PMID: 37353543 Free PMC article.
-
Mapping brain activity of gut-brain signaling to appetite and satiety in healthy adults: A systematic review and functional neuroimaging meta-analysis.Neurosci Biobehav Rev. 2022 May;136:104603. doi: 10.1016/j.neubiorev.2022.104603. Epub 2022 Mar 9. Neurosci Biobehav Rev. 2022. PMID: 35276299 Free PMC article.
-
An Emerging Role for Gut-Brain Signaling Involving Ghrelin in Chronic Stress.Adv Exp Med Biol. 2025;1477:205-227. doi: 10.1007/978-3-031-89525-8_7. Adv Exp Med Biol. 2025. PMID: 40442387 Review.
-
Combined Loss of Ghrelin Receptor and Cannabinoid CB1 Receptor in Mice Decreases Survival but does not Additively Reduce Body Weight or Eating.Neuroscience. 2020 Nov 1;447:53-62. doi: 10.1016/j.neuroscience.2019.09.005. Epub 2019 Sep 12. Neuroscience. 2020. PMID: 31520709 Free PMC article.
References
-
- Cameron JD Goldfield GS Cyr MJ Doucet E.. The effects of prolonged caloric restriction leading to weight-loss on food hedonics and reinforcement. Physiol Behav 2008;94:474–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

