Cav1.2 and Cav1.3 L-type calcium channels operate in a similar voltage range but show different coupling to Ca(2+)-dependent conductances in hippocampal neurons
- PMID: 24760982
- PMCID: PMC4059997
- DOI: 10.1152/ajpcell.00329.2013
Cav1.2 and Cav1.3 L-type calcium channels operate in a similar voltage range but show different coupling to Ca(2+)-dependent conductances in hippocampal neurons
Abstract
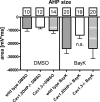
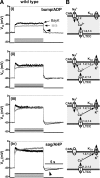
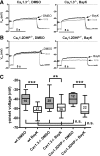
In the central nervous system, L-type voltage-gated calcium channels (LTCCs) come in two isoforms, namely Cav1.2 and Cav1.3 channels. It has been shown previously that these channels differ in biophysical properties, in subcellular localization, and in the coupling to the gene transcription machinery. In previous work on rat hippocampal neurons we have identified an excitatory cation conductance and an inhibitory potassium conductance as important LTCC coupling partners. Notably, a stimulus-dependent interplay of LTCC-mediated Ca(2+) influx and activation of these Ca(2+)-dependent conductances was found to give rise to characteristic voltage responses. However, the contribution of Cav1.2 and Cav1.3 to these voltage responses remained unknown. Hence, the relative contribution of the LTCC isoforms therein was the focus of the current study on hippocampal neurons derived from genetically modified mice, which either lack a LTCC isoform (Cav1.3 knockout mice) or express a dihydropyridine-insensitive LTCC isoform (Cav1.2DHP(-)-knockin mice). We identified common and alternate ion channel couplings of Cav1.2 and Cav1.3 channels. Whereas hyperpolarizing Ca(2+)-dependent conductances were coupled to both Cav1.2 and Cav1.3 channels, an afterdepolarizing potential was only induced by the activity of Cav1.2 channels. Unexpectedly, the activity of Cav1.2 channels was found at relatively hyperpolarized membrane voltages. Our data add important information about the differences between Cav1.2 and Cav1.3 channels that furthers our understanding of the physiological and pathophysiological neuronal roles of these calcium channels. Moreover, our findings suggest that Cav1.3 knockout mice together with Cav1.2DHP(-)-knockin mice provide valuable models for future investigation of hippocampal LTCC-dependent afterdepolarizations.
Keywords: dihydropyridine; gene deletion; knockout; voltage-gated calcium channel.
Copyright © 2014 the American Physiological Society.
Figures






References
-
- Amano H, Amano T, Matsubayashi H, Ishihara K, Serikawa T, Sasa M. Enhanced calcium influx in hippocampal CA3 neurons of spontaneously epileptic rats. Epilepsia 42: 345–350, 2001 - PubMed
-
- Barad M. Later developments: molecular keys to age-related memory impairment. Alzheimer Dis Assoc Disord 17: 168–176, 2003 - PubMed
-
- Cai X, Liang CW, Muralidharan S, Kao JP, Tang CM, Thompson SM. Unique roles of SK and Kv4.2 potassium channels in dendritic integration. Neuron 44: 351–364, 2004 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

