P2Y2 nucleotide receptor activation enhances the aggregation and self-organization of dispersed salivary epithelial cells
- PMID: 24760984
- PMCID: PMC4080185
- DOI: 10.1152/ajpcell.00380.2013
P2Y2 nucleotide receptor activation enhances the aggregation and self-organization of dispersed salivary epithelial cells
Abstract
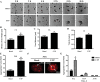
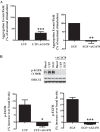
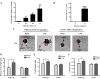
Hyposalivation resulting from salivary gland dysfunction leads to poor oral health and greatly reduces the quality of life of patients. Current treatments for hyposalivation are limited. However, regenerative medicine to replace dysfunctional salivary glands represents a revolutionary approach. The ability of dispersed salivary epithelial cells or salivary gland-derived progenitor cells to self-organize into acinar-like spheres or branching structures that mimic the native tissue holds promise for cell-based reconstitution of a functional salivary gland. However, the mechanisms involved in salivary epithelial cell aggregation and tissue reconstitution are not fully understood. This study investigated the role of the P2Y2 nucleotide receptor (P2Y2R), a G protein-coupled receptor that is upregulated following salivary gland damage and disease, in salivary gland reconstitution. In vitro results with the rat parotid acinar Par-C10 cell line indicate that P2Y2R activation with the selective agonist UTP enhances the self-organization of dispersed salivary epithelial cells into acinar-like spheres. Other results indicate that the P2Y2R-mediated response is dependent on epidermal growth factor receptor activation via the metalloproteases ADAM10/ADAM17 or the α5β1 integrin/Cdc42 signaling pathway, which leads to activation of the MAPKs JNK and ERK1/2. Ex vivo data using primary submandibular gland cells from wild-type and P2Y2R(-/-) mice confirmed that UTP-induced migratory responses required for acinar cell self-organization are mediated by the P2Y2R. Overall, this study suggests that the P2Y2R is a promising target for salivary gland reconstitution and identifies the involvement of two novel components of the P2Y2R signaling cascade in salivary epithelial cells, the α5β1 integrin and the Rho GTPase Cdc42.
Keywords: Cdc42 Rho GTPase; EGF receptor; P2Y2 nucleotide receptor; extracellular ATP; salivary gland reconstitution; α5β1 integrin.
Copyright © 2014 the American Physiological Society.
Figures



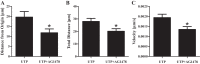

Similar articles
-
Therapeutic potential for P2Y2 receptor antagonism.Purinergic Signal. 2023 Jun;19(2):401-420. doi: 10.1007/s11302-022-09900-3. Epub 2022 Oct 11. Purinergic Signal. 2023. PMID: 36219327 Free PMC article. Review.
-
P2Y2 Nucleotide Receptor Prompts Human Cardiac Progenitor Cell Activation by Modulating Hippo Signaling.Circ Res. 2017 Nov 10;121(11):1224-1236. doi: 10.1161/CIRCRESAHA.117.310812. Epub 2017 Sep 18. Circ Res. 2017. PMID: 28923792 Free PMC article.
-
P2Y2 nucleotide receptor activation up-regulates vascular cell adhesion molecule-1 [corrected] expression and enhances lymphocyte adherence to a human submandibular gland cell line.Mol Immunol. 2008 Jan;45(1):65-75. doi: 10.1016/j.molimm.2007.05.009. Epub 2007 Jun 27. Mol Immunol. 2008. PMID: 17599409 Free PMC article.
-
P2Y2 nucleotide receptors mediate metalloprotease-dependent phosphorylation of epidermal growth factor receptor and ErbB3 in human salivary gland cells.J Biol Chem. 2010 Mar 5;285(10):7545-55. doi: 10.1074/jbc.M109.078170. Epub 2010 Jan 11. J Biol Chem. 2010. PMID: 20064929 Free PMC article.
-
Epithelial stem/progenitor cells in the embryonic mouse submandibular gland.Front Oral Biol. 2010;14:90-106. doi: 10.1159/000313709. Epub 2010 Apr 20. Front Oral Biol. 2010. PMID: 20428013 Free PMC article. Review.
Cited by
-
Therapeutic potential for P2Y2 receptor antagonism.Purinergic Signal. 2023 Jun;19(2):401-420. doi: 10.1007/s11302-022-09900-3. Epub 2022 Oct 11. Purinergic Signal. 2023. PMID: 36219327 Free PMC article. Review.
-
Pharmacological characterization of P2Y receptor subtypes - an update.Purinergic Signal. 2024 Apr;20(2):99-108. doi: 10.1007/s11302-023-09963-w. Epub 2023 Sep 12. Purinergic Signal. 2024. PMID: 37697211 Free PMC article. Review.
-
P2Y2 Nucleotide Receptor Prompts Human Cardiac Progenitor Cell Activation by Modulating Hippo Signaling.Circ Res. 2017 Nov 10;121(11):1224-1236. doi: 10.1161/CIRCRESAHA.117.310812. Epub 2017 Sep 18. Circ Res. 2017. PMID: 28923792 Free PMC article.
-
P2Y2 R deletion ameliorates sialadenitis in IL-14α-transgenic mice.Oral Dis. 2018 Jul;24(5):761-771. doi: 10.1111/odi.12823. Epub 2018 Mar 13. Oral Dis. 2018. PMID: 29297959 Free PMC article.
-
P2Y2 receptors mediate nucleotide-induced EGFR phosphorylation and stimulate proliferation and tumorigenesis of head and neck squamous cell carcinoma cell lines.Oral Oncol. 2020 Oct;109:104808. doi: 10.1016/j.oraloncology.2020.104808. Epub 2020 Jun 12. Oral Oncol. 2020. PMID: 32540611 Free PMC article.
References
-
- Ahn JS, Camden JM, Schrader AM, Redman RS, Turner JT. Reversible regulation of P2Y2 nucleotide receptor expression in the duct-ligated rat submandibular gland. Am J Physiol Cell Physiol 279: C286–C294, 2000. - PubMed
-
- Atkinson J, Fox P. Salivary gland dysfunction. Clin Geriatr Med 8: 499–511, 1992. - PubMed
-
- Atkinson JC, Baum BJ. Salivary enhancement: current status and future therapies. J Dent Educ 65: 1096–1101, 2001. - PubMed
-
- Atkinson JC, Wu AJ. Salivary gland dysfunction: causes, symptoms, treatment. J Am Dent Assoc 125: 409–416, 1994. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

