Conformational Substates of Myoglobin Intermediate Resolved by Picosecond X-ray Solution Scattering
- PMID: 24761190
- PMCID: PMC3985870
- DOI: 10.1021/jz4027425
Conformational Substates of Myoglobin Intermediate Resolved by Picosecond X-ray Solution Scattering
Abstract
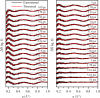
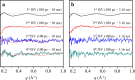
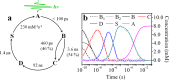
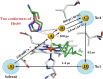
Conformational substates of proteins are generally considered to play important roles in regulating protein functions, but an understanding of how they influence the structural dynamics and functions of the proteins has been elusive. Here, we investigate the structural dynamics of sperm whale myoglobin associated with the conformational substates using picosecond X-ray solution scattering. By applying kinetic analysis considering all of the plausible candidate models, we establish a kinetic model for the entire cycle of the protein transition in a wide time range from 100 ps to 10 ms. Four structurally distinct intermediates are formed during the cycle, and most importantly, the transition from the first intermediate to the second one (B → C) occurs biphasically. We attribute the biphasic kinetics to the involvement of two conformational substates of the first intermediate, which are generated by the interplay between the distal histidine and the photodissociated CO.
Figures
References
-
- Frauenfelder H.; Sligar S. G.; Wolynes P. G. The Energy Landscapes and Motions of Proteins. Science 1991, 254, 1598–1603. - PubMed
-
- Mizutani Y.; Kitagawa T. Ultrafast Structural Relaxation of Myoglobin Following Photodissociation of Carbon Monoxide Probed by Time-Resolved Resonance Raman Spectroscopy. J. Phys. Chem. B 2001, 105, 10992–10999.
-
- Nishihara Y.; Sakakura M.; Kimura Y.; Terazima M. The Escape Process of Carbon Monoxide from Myoglobin to Solution at Physiological Temperature. J. Am. Chem. Soc. 2004, 126, 11877–11888. - PubMed
-
- Ahn S.; Kim K. H.; Kim Y.; Kim J.; Ihee H. Protein Tertiary Structural Changes Visualized by Time-Resolved X-ray Solution Scattering. J. Phys. Chem. B 2009, 113, 13131–13133. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

