Inhibition of Rgs10 Expression Prevents Immune Cell Infiltration in Bacteria-induced Inflammatory Lesions and Osteoclast-mediated Bone Destruction
- PMID: 24761229
- PMCID: PMC3994128
- DOI: 10.4248/BR201303005
Inhibition of Rgs10 Expression Prevents Immune Cell Infiltration in Bacteria-induced Inflammatory Lesions and Osteoclast-mediated Bone Destruction
Abstract
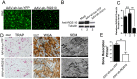
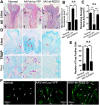
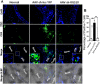
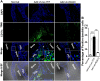
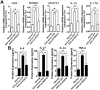
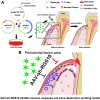
Regulator of G-protein Signaling 10 (Rgs10) plays an important function in osteoclast differentiation. However, the role of Rgs10 in immune cells and inflammatory responses, which activate osteoclasts in inflammatory lesions, such as bacteria-induced periodontal disease lesions, remains largely unknown. In this study, we used an adeno-associated virus (AAV-) mediated RNAi (AAV-shRNA-Rgs10) knockdown approach to study Rgs10's function in immune cells and osteoclasts in bacteria-induced inflammatory lesions in a mouse model of periodontal disease. We found that AAV-shRNA-Rgs10 mediated Rgs10 knockdown impaired osteoclastogenesis and osteoclast-mediated bone resorption, in vitro and in vivo. Interestingly, local injection of AAV-shRNA-Rgs10 into the periodontal tissues in the bacteria-induced inflammatory lesion greatly decreased the number of dendritic cells, T-cells and osteoclasts, and protected the periodontal tissues from local inflammatory damage and bone destruction. Importantly, AAV-mediated Rgs10 knockdown also reduced local expression of osteoclast markers and pro-inflammatory cytokines. Our results demonstrate that AAV-shRNA-Rgs10 knockdown in periodontal disease tissues can prevent bone resorption and inflammation simultaneously. Our data indicate that Rgs10 may regulate dendritic cell proliferation and maturation, as well as the subsequent stimulation of T-cell proliferation and maturation, and osteoclast differentiation and activation. Our study suggests that AAV-shRNA-Rgs10 can be useful as a therapeutic treatment of periodontal disease.
Keywords: AAV-mediated RNAi knockdown; Rgs10; bone resorption; gene therapy; gingival inflammation; immune cell; periodontal disease.
Figures

References
-
- Chambrone L, Guglielmetti MR, Pannuti CM, Chambrone LA. Evidence grade associating periodontitis to preterm birth and/or low birth weight: I. A systematic review of prospective cohort studies. J Clin Periodontol. 2011;38:795–808. - PubMed
-
- Van Dyke TE, Serhan CN. Resolution of inflammation: a new paradigm for the pathogenesis of periodontal diseases. J Dent Res. 2003;82:82–90. - PubMed
-
- D'Aiuto F, Parkar M, Andreou G, Suvan J, Brett PM, Ready D, Tonetti MS. Periodontitis and systemic inflammation: control of the local infection is associated with a reduction in serum inflammatory markers. J Dent Res. 2004;83:156–160. - PubMed
-
- Loe H. Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care. 1993;16:329–334. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

